Nucleus stability: Difference between revisions
(new article) |
(new article) |
||
| Line 1: | Line 1: | ||
{{Check|}} | |||
'''Nucleus stability, nuclide valley''' | '''Nucleus stability, nuclide valley''' | ||
Revision as of 15:26, 8 December 2013
|
Check of this article is requested. |
Nucleus stability, nuclide valley
What is the nuclear stability? Nuclear stability means that nucleus is stable meaning that it does not spontaneously emit any kind of radiation. On the other hand, if the nucleus is unstable, it has the tendency of emitting some kind of radiation, which makes it radioactive. Therefore the radioactivity is associated with unstable nucleus:
Stable nucleus – non-radioactive
Unstable nucleus – radioactive
We want to know why there is a radioactivity. What makes the nucleus stable? There are no concrete theories to explain this, but there are only general observations based on the available stable isotopes. It appears that neutron to proton ratio is the dominant factor in nuclear stability. Then how do we predict the nuclear stability? One of the simplest ways of predicting the nuclear stability is based on whether nucleus contains odd/even number of protons and neutrons:
| Protons | Neutrons | Number of Stable Nuclides | Stability |
|---|---|---|---|
| Odd | Odd | 4 | least stable |
| Odd | Example | 50 | less stable |
| Even | Odd | 57 | more stable |
| Even | Even | 168 | most stable |
• Nuclides containing odd numbers of both protons and neutrons are the least stable means more radioactive.
• Nuclides containing even numbers of both protons and neutrons are most stable means less radioactive.
• Nuclides contain odd numbers of protons and even numbers of neutrons are less stable than nuclides containing even numbers of protons and odd numbers of neutrons.
The Valley of Nuclear Stability
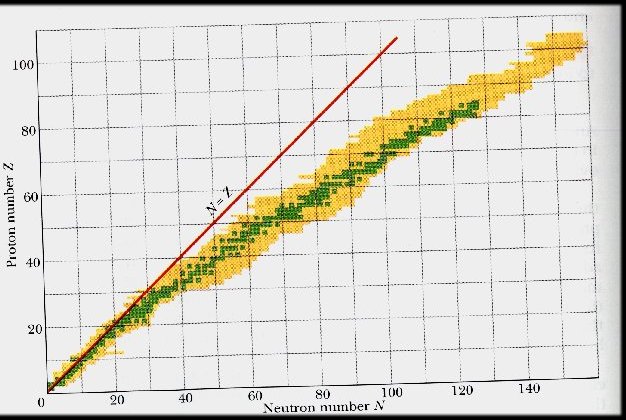
Number of protons and neutrons in nuclei
green square = stable nucleus
yellow square = radioactive nucleus
no square = no known nucleus
• Easier to put neutrons (0 charge) together than protons (+ charge repel)
• The bigger the nucleus, the more neutrons you can put in between protons
Reference http://faculty.ncc.edu/LinkClick.aspx?fileticket=sSPFB6xEtbM%3D&tabid=1918



