AIDS diagnosis: Difference between revisions
No edit summary |
(checked by the editor) |
||
| (19 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Standard aids diagnosis == | == Standard aids diagnosis == | ||
A single multi-test algorithm is used to diagnose [[HIV|human immunodeficiency virus (HIV)]] infection causing acquired [[AIDS|immune deficiency syndrome (AIDS)]]. The basis is an [[ELISA]] (Enzyme-linked Immunosorbent Assay) test supplemented by [[Southern blotting#Western blot protein analysis|western blot]]. | A single multi-test algorithm is used to diagnose [[HIV|human immunodeficiency virus (HIV)]] infection causing acquired [[AIDS|immune deficiency syndrome (AIDS)]]. The basis is an [[ELISA]] (Enzyme-linked Immunosorbent Assay) test supplemented by [[Southern blotting#Western blot protein analysis|western blot]]. In case of problems with the diagnosis or with the patient, a whole range of other tests is prepared to help confirm or refute the HIV infection. In case of problems with the diagnosis or with the patient, a whole range of other tests is prepared to help confirm or refute the HIV infection. | ||
=== ELISA test === | === ELISA test === | ||
The basis of testing for the presence of HIV in the body is the detection of [[antibody|antibodies]] | The basis of testing for the presence of HIV in the body is the detection of [[antibody|antibodies]] in serum or other body fluids. ELISA is one of the methods for detecting the patient's production of antibodies to the virus. The specificity and sensitivity of this test is more than 99%. Therefore, the ELISA is used in most cases as the first test in case of suspected HIV infection. | ||
If the test is '''negative''', there is no reason to suspect HIV infection and the patient is considered healthy. However, there may be situations where the patient has recently become infected, resulting in a ''window period''. Although the infection is present in the body, detectable amounts of antibodies have not yet begun to form. The diagnostic window can last up to 12 weeks (depending on the ELISA type). The ELISA may also be false negative in cases of [[autoimmune disease|autoimmune disease]], [[Renal failure|renal failure]], [[hemodialysis|hemodialysis]], [[cystic fibrosis|cystic fibrosis]], multiple pregnancies or transfusions, and liver disease. | |||
If the test is '''positive''', the ELISA is repeated because, of course, the test may be false-positive. The causes are the same as for a false-negative test, and false-positive results can also be obtained with intravenous drug use, vaccination ([[hepatitis]], [[rabies]],…) or [[HIV vaccination|HIV vaccination]] as part of the research. If the second ELISA test is positive again, further testing (western blot) is performed for a high probability of infection. If the results of both tests are different (positive and negative), it is tested again. It is advantageous to perform tests with samples taken by the same method and evaluated in the same laboratory. | |||
=== Western blot === | === Western blot === | ||
[[ | [[Souther blotting#Western blot protein analysis|Western blot]] detects the presence of antibodies against [[virus]] proteins: core-proteins (p17, p24, p55), polymerases (p31, p51, p66) a envelope-proteins (gp41, gp120, gp160). The presence of certain groups of antibodies is always evaluated. If only some antibodies are present that do not meet the criteria of the positive test, the test result is marked as indeterminate. | ||
''' | '''A positive''' Western blot in combination with a positive [[ELISA]] test already means the almost certain presence of [[HIV]], the patient is HIV positive and [[treatment ]]can start. The specificity of the western blot is 97.8%, which means that more than 2% of the tests are false positive. The error may be due to sample swapping, contamination of negative samples with positive samples, or antibodies to [[major histocompatibility complex|HLA antigenům]] antigens in the virus lysate used in the assay, and misinterpretation of electrophoretic bands from the sample. | ||
''' | '''A negative result''' is considered to be no [[infection]] and the patient is, therefore, HIV negative. However, re-testing is recommended for patients at high risk of infection, as with the [[ELISA]] the patient may be infected recently and tested in the diagnostic window. | ||
''' | '''The indeterminate result''' can have many causes. Testing in the diagnostic window will cause some [[antibodies]] to be not detectable in the sample. Performing a test in the late phase of HIV infection may result in failure to capture antibodies to the virus's core proteins. Some healthy people may produce antibodies that cross-react with specific HIV-1 peptides or recombinant [[antigens]] for no apparent reason. | ||
If the patient is at high risk for HIV infection, it is recommended that they be monitored for at least 6 months. For clients with a low probability of infection, we can consider the test as negative, but also with the possibility of further monitoring. In the case of an indeterminate western blot result, it is time to test the amount of viral [[RNA]] present in the plasma: Plasma Viral Load. | |||
=== Plasma Viral Load === | === Plasma Viral Load === | ||
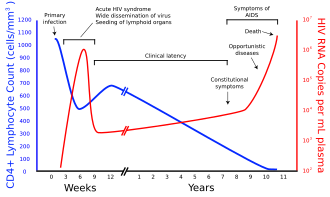
Plasma Viral Load | [[File:Hiv-timecourse copy.svg|thumb|Graph showing HIV copies and CD4 counts in a human over the course of a treatment-naive HIV infection]] | ||
Plasma Viral Load is a set of tests that quantitatively detect the presence of viral RNA in a patient's plasma. It consists of three parts: | |||
* '''[[ | * '''[[Polymerase Chain Reaction|PCR]] assay''' – Polymerase Chain Reaction Assay; | ||
* '''[[DNA ( | * '''[[DNA (nucleic acid)|bDNA]] assay''' – Branched-DNA Assay; | ||
* '''NASBA''' – Nucleic Acid Sequence-Based Amplification. | * '''NASBA''' – Nucleic Acid Sequence-Based Amplification. | ||
The most common reasons for Plasma Viral Load (PVL) are: | |||
* | * there is a reasonable suspicion of [[HIV]], infection or the patient is showing signs of a viral infection; | ||
* | * vague [[HIV antibody]] test results in a patient at high risk of infection; | ||
* | * confirmation of a newly diagnosed HIV infection; | ||
* | * observation of patients without antiviral treatment; | ||
* test | * a test before starting or changing antiviral treatment; | ||
* | * testing during treatment to compare results. | ||
During the [[ELISA]] a [[Southern blotting#Western blot protein analysis|western blot]], diagnostic window, when [[antibodies]], have not yet formed, a certain amount of viral RNA can be captured in the plasma. The sensitivity limit can be as high as 20-50 RNA molecules per millilitre of plasma. However, the PVL test is used as an initial one only rarely, because its price is much higher than for other standardized tests. | |||
To determine the degree of the disease, the number of [[T-lymphocytes|CD4+ T-lymphocytes]] in the blood is also determined, most often by [[flow cytometry|flow cytometry]]. This examination also serves to control the effects of antiretroviral treatment. New tests show that in areas without the possibility of rapid transport of chilled and anticoagulated blood to the laboratory, it will probably be possible to use dried blood smears treated with EIA [[EIA (Enzyme Immunoassay)]] for CD4 + cell counting and Plasma Viral Load. | |||
== | == New methods of HIV detection == | ||
According to studies conducted in 1995 in the USA, about 25% of HIV-positive and 33% of HIV-negative clients did not show up for HIV results after HIV testing. The problem is not just developed countries. In developing countries, there is a difficult possibility of laboratory testing - correct and fast transport of samples, lack of equipment, etc. It was, therefore, necessary to ensure fast, convenient, cheap and yet reliable methods to implement HIV testing. | |||
=== | === Fast testing === | ||
Quick tests can be completed in as little as 30 minutes. They include a test for HIV-1 and HIV-2 antibodies using various carriers (latex agglutination, immunoassay,…). These tests are used, for example, in North African countries as an alternative to ELISA and western blot. Rapid tests have a specificity of more than 96% and a sensitivity of about 99% at a low testing cost. | |||
=== | === RNA amplification === | ||
This method is currently in the research phase. There are several different commercial test kits that are tested on volunteers. Viral nucleic acid amplification allows the diagnostic window to be shortened to up to 8 days. | |||
=== | === Home test kits === | ||
Home test kits allow you to easily take a blood sample and send a special filter paper with a sample marked only with an anonymous number to the laboratory. The client is informed of the result by telephone and is provided with a basic consultation together with recommendations for further action. | |||
=== | === Non-invasive tests === | ||
Non-invasive tests include the collection of transudate from the oral mucosa, urine and vaginal secretions. ELISA is used for sample analysis. The detection capability is, of course, lower than with conventional methods, but the advantages of non-invasive testing include patient comfort and also a lower risk of complications for both the patient and the healthcare professional. Said body fluids contain [[IgG]] antibodies which can be detected by an ELISA test. | |||
== | == HIV testing in blood donors == | ||
HIV testing is not just about patients, but also about blood donors. In the Czech Republic, according to the Decree on Human Blood 143/2008 Coll. each blood sample must be tested for HIV-1 and HIV-2 infection by [[antibody]] and p24 antigen. This minimizes the risk of HIV transmission from the infected blood donor to the recipient. | |||
<noinclude> | <noinclude> | ||
== | |||
=== | == References == | ||
=== Related articles === | |||
* [[HIV]] | * [[HIV]] | ||
* [[AIDS]] | * [[AIDS]] | ||
* [[Epidemiologie AIDS]] | * [[Epidemiologie AIDS]] | ||
=== | === References === | ||
* {{ | * {{Cite | ||
| | |type = article | ||
| | |corporation = | ||
| | |surname1 = Cheng | ||
| | |name1 = B. | ||
| | |surname2 = Landay | ||
| | |name2 = A. | ||
| | |surname3 = Miller | ||
| | |name3 = V | ||
| | |others = no | ||
| | |article = Research needs and challenges in the development of HIV diagnostic and treatment monitoring tests for use in resource-limited settings | ||
| | |journal = Current Opinion in HIV and AIDS | ||
| | |year = July 2008 | ||
| | |the_year = | ||
| | |volume = 3 | ||
| | |number = | ||
| | |pages = 495-503 | ||
|issn = 1746-630X | |issn = 1746-630X | ||
|url = http://ovidsp.tx.ovid.com/sp-3.5.1a/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&AN=01222929-200807000-00014&NEWS=N&CSC=Y&CHANNEL=PubMed | |url = http://ovidsp.tx.ovid.com/sp-3.5.1a/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&AN=01222929-200807000-00014&NEWS=N&CSC=Y&CHANNEL=PubMed | ||
}} | }} | ||
* {{ | * {{Cite | ||
| | |type = article | ||
| | |corporation = | ||
| | |surname1 = Mylonakis | ||
| | |name1 = E. | ||
| | |surname2 = Paliou | ||
| | |name2 = M. | ||
| | |surname3 = Lally | ||
| | |name3 = M. | ||
| | |others = yes | ||
| | |article = Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches | ||
| | |journal = The American Journal of Medicine | ||
| | |year = Nov 2000 | ||
| | |the_year = | ||
| | |volume = 109 | ||
| | |number = | ||
| | |pages = 568-576 | ||
|issn = 0002-9343 | |issn = 0002-9343 | ||
|url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TDC-41JKV3D-8&_user=1490772&_coverDate=11%2F30%2F2000&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000053052&_version=1&_urlVersion=0&_userid=1490772&md5=4ffa42c0a536d1329d5f61c401284f41 | |url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TDC-41JKV3D-8&_user=1490772&_coverDate=11%2F30%2F2000&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000053052&_version=1&_urlVersion=0&_userid=1490772&md5=4ffa42c0a536d1329d5f61c401284f41 | ||
}} | }} | ||
* {{ | * {{Cite | ||
| | |type = article | ||
| | |corporation = | ||
| | |surname1 = Mylonakis | ||
| | |name1 = E. | ||
| | |surname2 = Paliou | ||
| | |name2 = M. | ||
| | |surname3 = Rich | ||
| | |name3 = J | ||
| | |others = no | ||
| | |article = Plasma Viral Load Testing in the Management of HIV Infection | ||
| | |journal = American Family Physician | ||
| | |year = Feb 2001 | ||
| | |the_year = | ||
| | |volume = 63 | ||
| | |number = | ||
| | |pages = 483-490 | ||
|issn = 0002-838X | |issn = 0002-838X | ||
|url = https://www.aafp.org/afp/2001/0201/p483.html | |url = https://www.aafp.org/afp/2001/0201/p483.html | ||
}} | }} | ||
* {{ | * {{Cite | ||
| | |type = article | ||
| | |corporation = | ||
| | |surname1 = Masopust | ||
| | |name1 = J. | ||
| | |surname2 = Ročková | ||
| | |name2 = R | ||
| | |surname3 = | ||
| | |name3 = | ||
| | |others = no | ||
| | |article = Význam rozšíření screeningového vyšetření HIV infekce u dárců krve a krevních složek v České republice | ||
| | |journal = Transfuze a hematologie dnes | ||
| | |year = 2003 | ||
| | |the_year = | ||
| | |volume = 2 | ||
| | |number = | ||
| | |pages = 59-64 | ||
|issn = - | |issn = - | ||
|url = | |url = | ||
| Line 152: | Line 150: | ||
</noinclude> | </noinclude> | ||
[[Category: | [[Category:Microbiology]] | ||
[[Category: | [[Category:Viruses]] | ||
[[Category: | [[Category:Infectious medicine]] | ||
[[Category: | [[Category:Epidemiology]] | ||
Latest revision as of 18:07, 31 December 2021
Standard aids diagnosis[edit | edit source]
A single multi-test algorithm is used to diagnose human immunodeficiency virus (HIV) infection causing acquired immune deficiency syndrome (AIDS). The basis is an ELISA (Enzyme-linked Immunosorbent Assay) test supplemented by western blot. In case of problems with the diagnosis or with the patient, a whole range of other tests is prepared to help confirm or refute the HIV infection. In case of problems with the diagnosis or with the patient, a whole range of other tests is prepared to help confirm or refute the HIV infection.
ELISA test[edit | edit source]
The basis of testing for the presence of HIV in the body is the detection of antibodies in serum or other body fluids. ELISA is one of the methods for detecting the patient's production of antibodies to the virus. The specificity and sensitivity of this test is more than 99%. Therefore, the ELISA is used in most cases as the first test in case of suspected HIV infection.
If the test is negative, there is no reason to suspect HIV infection and the patient is considered healthy. However, there may be situations where the patient has recently become infected, resulting in a window period. Although the infection is present in the body, detectable amounts of antibodies have not yet begun to form. The diagnostic window can last up to 12 weeks (depending on the ELISA type). The ELISA may also be false negative in cases of autoimmune disease, renal failure, hemodialysis, cystic fibrosis, multiple pregnancies or transfusions, and liver disease.
If the test is positive, the ELISA is repeated because, of course, the test may be false-positive. The causes are the same as for a false-negative test, and false-positive results can also be obtained with intravenous drug use, vaccination (hepatitis, rabies,…) or HIV vaccination as part of the research. If the second ELISA test is positive again, further testing (western blot) is performed for a high probability of infection. If the results of both tests are different (positive and negative), it is tested again. It is advantageous to perform tests with samples taken by the same method and evaluated in the same laboratory.
Western blot[edit | edit source]
Western blot detects the presence of antibodies against virus proteins: core-proteins (p17, p24, p55), polymerases (p31, p51, p66) a envelope-proteins (gp41, gp120, gp160). The presence of certain groups of antibodies is always evaluated. If only some antibodies are present that do not meet the criteria of the positive test, the test result is marked as indeterminate.
A positive Western blot in combination with a positive ELISA test already means the almost certain presence of HIV, the patient is HIV positive and treatment can start. The specificity of the western blot is 97.8%, which means that more than 2% of the tests are false positive. The error may be due to sample swapping, contamination of negative samples with positive samples, or antibodies to HLA antigenům antigens in the virus lysate used in the assay, and misinterpretation of electrophoretic bands from the sample.
A negative result is considered to be no infection and the patient is, therefore, HIV negative. However, re-testing is recommended for patients at high risk of infection, as with the ELISA the patient may be infected recently and tested in the diagnostic window.
The indeterminate result can have many causes. Testing in the diagnostic window will cause some antibodies to be not detectable in the sample. Performing a test in the late phase of HIV infection may result in failure to capture antibodies to the virus's core proteins. Some healthy people may produce antibodies that cross-react with specific HIV-1 peptides or recombinant antigens for no apparent reason.
If the patient is at high risk for HIV infection, it is recommended that they be monitored for at least 6 months. For clients with a low probability of infection, we can consider the test as negative, but also with the possibility of further monitoring. In the case of an indeterminate western blot result, it is time to test the amount of viral RNA present in the plasma: Plasma Viral Load.
Plasma Viral Load[edit | edit source]
Plasma Viral Load is a set of tests that quantitatively detect the presence of viral RNA in a patient's plasma. It consists of three parts:
- PCR assay – Polymerase Chain Reaction Assay;
- bDNA assay – Branched-DNA Assay;
- NASBA – Nucleic Acid Sequence-Based Amplification.
The most common reasons for Plasma Viral Load (PVL) are:
- there is a reasonable suspicion of HIV, infection or the patient is showing signs of a viral infection;
- vague HIV antibody test results in a patient at high risk of infection;
- confirmation of a newly diagnosed HIV infection;
- observation of patients without antiviral treatment;
- a test before starting or changing antiviral treatment;
- testing during treatment to compare results.
During the ELISA a western blot, diagnostic window, when antibodies, have not yet formed, a certain amount of viral RNA can be captured in the plasma. The sensitivity limit can be as high as 20-50 RNA molecules per millilitre of plasma. However, the PVL test is used as an initial one only rarely, because its price is much higher than for other standardized tests.
To determine the degree of the disease, the number of CD4+ T-lymphocytes in the blood is also determined, most often by flow cytometry. This examination also serves to control the effects of antiretroviral treatment. New tests show that in areas without the possibility of rapid transport of chilled and anticoagulated blood to the laboratory, it will probably be possible to use dried blood smears treated with EIA EIA (Enzyme Immunoassay) for CD4 + cell counting and Plasma Viral Load.
New methods of HIV detection[edit | edit source]
According to studies conducted in 1995 in the USA, about 25% of HIV-positive and 33% of HIV-negative clients did not show up for HIV results after HIV testing. The problem is not just developed countries. In developing countries, there is a difficult possibility of laboratory testing - correct and fast transport of samples, lack of equipment, etc. It was, therefore, necessary to ensure fast, convenient, cheap and yet reliable methods to implement HIV testing.
Fast testing[edit | edit source]
Quick tests can be completed in as little as 30 minutes. They include a test for HIV-1 and HIV-2 antibodies using various carriers (latex agglutination, immunoassay,…). These tests are used, for example, in North African countries as an alternative to ELISA and western blot. Rapid tests have a specificity of more than 96% and a sensitivity of about 99% at a low testing cost.
RNA amplification[edit | edit source]
This method is currently in the research phase. There are several different commercial test kits that are tested on volunteers. Viral nucleic acid amplification allows the diagnostic window to be shortened to up to 8 days.
Home test kits[edit | edit source]
Home test kits allow you to easily take a blood sample and send a special filter paper with a sample marked only with an anonymous number to the laboratory. The client is informed of the result by telephone and is provided with a basic consultation together with recommendations for further action.
Non-invasive tests[edit | edit source]
Non-invasive tests include the collection of transudate from the oral mucosa, urine and vaginal secretions. ELISA is used for sample analysis. The detection capability is, of course, lower than with conventional methods, but the advantages of non-invasive testing include patient comfort and also a lower risk of complications for both the patient and the healthcare professional. Said body fluids contain IgG antibodies which can be detected by an ELISA test.
HIV testing in blood donors[edit | edit source]
HIV testing is not just about patients, but also about blood donors. In the Czech Republic, according to the Decree on Human Blood 143/2008 Coll. each blood sample must be tested for HIV-1 and HIV-2 infection by antibody and p24 antigen. This minimizes the risk of HIV transmission from the infected blood donor to the recipient.
References[edit | edit source]
Related articles[edit | edit source]
References[edit | edit source]
- CHENG, B. – LANDAY, A. – MILLER, V. Research needs and challenges in the development of HIV diagnostic and treatment monitoring tests for use in resource-limited settings. Current Opinion in HIV and AIDS [online]. July 2008, vol. 3, p. 495-503, Available from <http://ovidsp.tx.ovid.com/sp-3.5.1a/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&AN=01222929-200807000-00014&NEWS=N&CSC=Y&CHANNEL=PubMed>. ISSN 1746-630X.
- MYLONAKIS, E. – PALIOU, M. – LALLY, M.. , et al. Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches. The American Journal of Medicine [online]. Nov 2000, vol. 109, p. 568-576, Available from <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TDC-41JKV3D-8&_user=1490772&_coverDate=11%2F30%2F2000&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000053052&_version=1&_urlVersion=0&_userid=1490772&md5=4ffa42c0a536d1329d5f61c401284f41>. ISSN 0002-9343.
- MYLONAKIS, E. – PALIOU, M. – RICH, J. Plasma Viral Load Testing in the Management of HIV Infection. American Family Physician [online]. Feb 2001, vol. 63, p. 483-490, Available from <https://www.aafp.org/afp/2001/0201/p483.html>. ISSN 0002-838X.
- MASOPUST, J. – ROČKOVÁ, R. Význam rozšíření screeningového vyšetření HIV infekce u dárců krve a krevních složek v České republice. Transfuze a hematologie dnes. 2003, vol. 2, p. 59-64,