Nutrient chemistry
Content of the subsection
- Nutrient overview – sacharides
- Nutrient overview – lipids
- Nutrient overview – proteins
Nutrient overview - sacharides
Clasification and structure
Sacharides, also called carbohydrates or glycids, are the most abundant organic substances on Earth. Their molecules are made up of oxygen, carbon and hydrogen atoms. From a chemical point of view, these are polyhydroxyaldehydes and polyhydroxyketones. They contain functional aldehyde or keto groups in their molecule, as well as a larger number of hydroxyl groups.
Clasification of carbohydrates
According to the number of units in the molecule, we distinguish:
- monosaccharides – cannot be further hydrolyzed into simpler units;
- oligosaccharides – they form 2–10 units of monosaccharides by hydrolysis;
- polysaccharides – hydrolyzing into more than 10 monosaccharides.
- Monosaccharides and oligosaccharides are generally called sugars. A synonym for polysaccharide is the word glycan.
We divide monosaccharides according to:
- Number of C-atoms: trioses, tetroses, pentoses, hexoses.
- Functional groups: aldoses and ketoses.
We divide polysaccharides into:
- Homopolysaccharides: these are polymers made up of the same type of monosaccharide. Examples are starch, glycogen or cellulose.
- Heteropolysaccharides: they are polymers made up of more than one type of monosaccharide. An example is hemicellulose.
Structure of saccharides
The structure of a saccharide molecule can be expressed in different formulas:
- Linear (Fischer) formula;
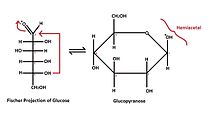
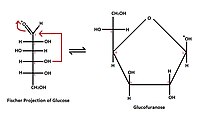
- Cyclic (Haworth) formula, which results from the formation of a heterocyclic structure.
- The cycle can contain:
- six atoms - pyranose - similar to six-carbon pyran;
- five atoms - furanose - similar to five-carbon furan.
- Tollens' formula describes the creation of a cyclic structure from a linear formula. It shows the reaction of hydroxyl with a carbonyl group to form a so-called hemiacetal structure.
Isomerism
It is a state where compounds with the same general formula have a different structural arrangement of atoms in the molecule. The following types of isomerism are found in carbohydrate molecules.
- D- and L- prefixes
- It is determined by the position of the −OH group on the last chiral carbon. Assignment of the prefix is based on similarity with the original compound of the carbohydrate series – glyceraldehyde. The −OH group is located on the right for D- and on the left for L- isomers in the Fischer formula.
- D- and L- isomers are mirror images - so-called enantiomers - optical isomers. They differ in the sign of optical rotation, or the direction in which they rotate the plane of polarized light.
- However, it is not generally the case that the D-isomers rotate light to the right and the L-isomers rotate light to the left.
- An equimolar mixture of enantiomers is called a racemic mixture, or a DL mixture, and does not exhibit optical activity.
- D-isomers are more common in nature.
- Pyranoses and furanoses
- They are labeled according to the similarity of the cyclic form of the respective monosaccharide with the pyran or furan cycle. Glucose in solution occurs more than 99% in the form of gluco-pyranose, the rest of the molecules, less than 1%, then appears in the form of gluco-furanose.
- α- a β- anomers
- They are labeled according to the position of the hemiacetal or hemiketal −OH in the cycle. Hemiacetals are formed by the reaction of aldehyde and alcohol groups, hemiketals by the reaction of keto and alcohol groups.
- If the −OH group is oriented to the same side as the −OH group indicating belonging to the D- or L- group, it is an α-anomer. If the −OH group is oriented to the opposite side, it is a β-anomer.
Anomers differ in optical rotation.
- Epimers
- hey differ from each other by the position of one −OH group in the molecule.
Examples are glucose and mannose.
- Aldoses a ketoses
- They are labeled according to the functional group on the 1st and 2nd carbons of the molecule.
Sacharidy nejsou pro tělo esenciální a běžně se v něm syntetizují, např. z aminokyselin nebo glycerolu.
- Monosacharidy a disacharidy představují důležitý zdroj energie. Jsou nezbytné zejména pro buňky mozku a erytrocyty.
- Polysacharidy slouží jako zásobárna energie – glykogen u živočichů.
Sacharidy plní i strukturní funkce, například jako součást glykoproteinů a glykolipidů v membránách.
Iron
Klíčovou roli hrají i při syntéze nukleových kyselin nebo koenzymů. Jsou též součástí mezibuněčné hmoty, například v molekulách proteoglykanů.