Nutrient chemistry
Content of the subsection[edit | edit source]
- Nutrient overview – sacharides
- Nutrient overview – lipids
- Nutrient overview – proteins
Nutrient overview - sacharides[edit | edit source]
Clasification and structure[edit | edit source]
Sacharides, also called carbohydrates or glycids, are the most abundant organic substances on Earth. Their molecules are made up of oxygen, carbon and hydrogen atoms. From a chemical point of view, these are polyhydroxyaldehydes and polyhydroxyketones. They contain functional aldehyde or keto groups in their molecule, as well as a larger number of hydroxyl groups.
Clasification of carbohydrates[edit | edit source]
According to the number of units in the molecule, we distinguish:
- monosaccharides – cannot be further hydrolyzed into simpler units;
- oligosaccharides – they form 2–10 units of monosaccharides by hydrolysis;
- polysaccharides – hydrolyzing into more than 10 monosaccharides.
- Monosaccharides and oligosaccharides are generally called sugars. A synonym for polysaccharide is the word glycan.
We divide monosaccharides according to:
- Number of C-atoms: trioses, tetroses, pentoses, hexoses.
- Functional groups: aldoses and ketoses.
We divide polysaccharides into:
- Homopolysaccharides: these are polymers made up of the same type of monosaccharide. Examples are starch, glycogen or cellulose.
- Heteropolysaccharides: they are polymers made up of more than one type of monosaccharide. An example is hemicellulose.
Structure of saccharides[edit | edit source]
The structure of a saccharide molecule can be expressed in different formulas:
- Linear (Fischer) formula;
- Cyclic (Haworth) formula, which results from the formation of a heterocyclic structure.
- The cycle can contain:
- six atoms - pyranose - similar to six-carbon pyran;
- five atoms - furanose - similar to five-carbon furan.
- Tollens' formula describes the creation of a cyclic structure from a linear formula. It shows the reaction of hydroxyl with a carbonyl group to form a so-called hemiacetal structure.
Isomerism[edit | edit source]
It is a state where compounds with the same general formula have a different structural arrangement of atoms in the molecule. The following types of isomerism are found in carbohydrate molecules.
- D- and L- prefixes
- It is determined by the position of the −OH group on the last chiral carbon. Assignment of the prefix is based on similarity with the original compound of the carbohydrate series – glyceraldehyde. The −OH group is located on the right for D- and on the left for L- isomers in the Fischer formula.
- D- and L- isomers are mirror images - so-called enantiomers - optical isomers. They differ in the sign of optical rotation, or the direction in which they rotate the plane of polarized light.
- However, it is not generally the case that the D-isomers rotate light to the right and the L-isomers rotate light to the left.
- An equimolar mixture of enantiomers is called a racemic mixture, or a DL mixture, and does not exhibit optical activity.
- D-isomers are more common in nature.
- Pyranoses and furanoses
- They are labeled according to the similarity of the cyclic form of the respective monosaccharide with the pyran or furan cycle. Glucose in solution occurs more than 99% in the form of gluco-pyranose, the rest of the molecules, less than 1%, then appears in the form of gluco-furanose.
- α- a β- anomers
- They are labeled according to the position of the hemiacetal or hemiketal −OH in the cycle. Hemiacetals are formed by the reaction of aldehyde and alcohol groups, hemiketals by the reaction of keto and alcohol groups.
- If the −OH group is oriented to the same side as the −OH group indicating belonging to the D- or L- group, it is an α-anomer. If the −OH group is oriented to the opposite side, it is a β-anomer.
Anomers differ in optical rotation.
- Epimers
- hey differ from each other by the position of one −OH group in the molecule.
Examples are glucose and mannose.
- Aldoses a ketoses
- They are labeled according to the functional group on the 1st and 2nd carbons of the molecule.
Carbohydrates are not essential for the body and are normally synthesized in it, e.g. from amino acids or glycerol.
- Monosaccharides and disaccharides represent an important source of energy. They are especially necessary for brain cells and erythrocytes.
- Polysaccharides serve as energy storage - glycogen in animals.
Carbohydrates also perform structural functions, for example as part of glycoproteins and glycolipids in membranes.
They also play a key role in the synthesis of nucleic acids or coenzymes. They are also part of the intercellular mass, for example in proteoglycan molecules.
Monosaccharides and disaccharides[edit | edit source]
- Monosaccharides and disaccharides are white crystalline substances soluble in water, of a neutral nature, which do not dissociate in aqueous solutions. They have a polar character and the −OH groups cause their sweet taste and strong hydration in solution.
- The most important monosaccharides in food are glucose, fructose and galactose. Of the disaccharides, sucrose α-Glc (1→2) β-Fru used as a sweetener, beet sugar, lactose, β-Gal (1→4) β-Glc, present in milk and maltose, α-Glc (1→4) β -Glc, present in malt.
Sugar alcohols[edit | edit source]
- Sugar alcohols are formed by the reduction of a carbonyl group to a hydroxyl group. For example, glucitol a.k.a. sorbitol, which is produced by the reduction of glucose or fructose.
Polyhydroxy derivatives of carboxylic acids[edit | edit source]
- They are formed by the oxidation of monosaccharides. When oxidized with a weak reagent, the aldehyde group is oxidized and aldonic acids are formed. Stronger reagents oxidize not only the aldehyde group, but also the primary −OH groups at the end of the molecule, so that dicarboxylic aldaric acids are formed. Oxidation of only the primary −OH group of aldoses in the body takes place enzymatically to form uronic acids. For example, glucose produces glucuronic acid, an important conjugation agent in the liver that aids in the excretion of poorly water-soluble substances.
Deoxysugars[edit | edit source]
- They are formed by the reduction of the hydroxyl group of a saccharide. An example is deoxyribose, an important component of nucleic acids.
Aminosugars[edit | edit source]
- They are formed by replacing the hydroxyl group with the −NH2 group. Important amino sugars in the body include, for example, D-glucosamine, a component of intercellular mass molecules.
Esters[edit | edit source]
- They are formed by esterification of the hydroxyl group of H3PO4. For example, the formation of glucose-6-phosphate from a glucose molecule. Or H2SO4 components of proteoglycans.
Glycosides[edit | edit source]
They are formed by the reaction of hydroxyl groups with:
- Alcohol – formation of an O-glycosidic bond. For example, the formation of di- and polysaccharides, or the binding of monosaccharides to proteins via the amino acids serine and threonine.
- Amine - formation of an N-glycosidic bond. An example is the binding to proteins via aspartate or the binding of ribose in nucleotides.
- The most reactive group in the monosaccharide molecule is the anomeric group −OH.
- Non-reducing disaccharides are formed when a glycosidic bond is formed between the anomeric hydroxyls of both monosaccharides, as for example with sucrose. The disaccharide does not react with the oxidizing agent.
- A reducing disaccharide is formed when the anomeric hydroxyl of one monosaccharide reacts with a non-anomeric hydroxyl of another monosaccharide. Free aldoses, monosaccharides, are all reducing, some examples of reducing disaccharides are lactose or maltose.
Polysaccharides and fiber[edit | edit source]
- Polysaccharides tend to be amorphous substances and are either insoluble in water or form colloidal solutions. They are generally referred to as glycans. They can be made of only one type of monosaccharide, for example glucose as in starch and glycogen. We refer to these polysaccharides as glucans. If the monosaccharide is fructose, we call this polysaccharide fructan. They can also be made up of various monosaccharides and their derivatives, such as glycosaminoglycans.
- Storage polysaccharides such as starch or glycogen are partially soluble in water, while structural polysaccharides such as cellulose have many intra- and intermolecular hydrogen bonds in their structure and are insoluble in water.
Fiber[edit | edit source]
- It consists of a heterogeneous group of structural polysaccharides that cannot be broken down by human enzymes, which is why it is a non-absorbable part of food. However, it is very important for digestion - it increases the volume of digestate, which accelerates intestinal peristalsis and thus harmful substances remain in the digestive tract for a shorter time. At the same time, it binds to itself some foreign and endogenous substances, thereby increasing their excretion from the organism. This applies, for example, to bile acids formed from cholesterol - consumption of fiber therefore reduces the amount of cholesterol in the body.
We divide the fiber:
- Soluble fiber (hemicellulose, pectins).
It is broken down by colon bacteria into short-chain fatty acids, acetic, propionic, butyric acid, which are an important source of energy for colonocytes.
- Insoluble fiber (cellulose).
Even bacterial enzymes cannot break down cellulose and it leaves the body undigested. Its importance lies in increasing the volume of digestion and supporting peristaltic movements.
Heteroglycosides[edit | edit source]
- Substances containing, in addition to the carbohydrate part, another type of compound are called aglycones. Includes:
- Proteoglycans
- They contain linear long polysaccharide chains bound to protein. The chains are made up of repeating amino sugar-uronic acid dimers – these are referred to as glycosaminoglycans (GAGs).
- Glycoproteins
- Unlike proteoglycans, glycoproteins, i.e. proteins glycosylated in various places (O– or N– glycosidic bond) by short branched molecules of oligosaccharides, do not contain uronic acids.
- Glycolipids
- Glycolipids, substances of a lipid nature, have one or several monosaccharide units in the molecule.
Nutrient overview - Lipids[edit | edit source]
Lipids, a group of chemically and functionally heterogeneous substances, have in common insolubility in water – hydrophobicity, excellent solubility in non-polar solvents and the presence of alcohols and fatty acids in the molecule. They are often synthesized in the body from acetyl-CoA.
Fatty acids usually mean higher monocarboxylic acids. They contain approximately 8 or more carbon atoms, with typically an even number of carbon atoms (because they are formed from acetyl-CoA). If they contain double bonds, they are usually isolated and in the cis-configuration. Acids with a length of C16 and C18 are predominant.
Clasification of lipids[edit | edit source]
Simple lipids[edit | edit source]
- Acylglyceroles
- Waxes
Complex lipids[edit | edit source]
- Phospholipids
- Glycerolphospholipids
- Sphingolipids
- Glycolipids
- Cerebrosides
- Gangliosides
- Lipoproteins
Simple lipids[edit | edit source]
Simple lipids are esters of fatty acids and alcohols. They consist only of a hydrophobic part.
Acylglycerols[edit | edit source]
Acylglycerols (glycerides) are esters of higher fatty acids and glycerol. According to the number of fatty acid molecules bound to alcohol, they are divided into mono-, di- or triacylglycerols. The most important for us are triacylglycerols, which are part of fats - a mixture of solid triacylglycerols, or oils - a mixture of liquid triacylglycerols.
Acid hydrolysis of acylglycerols produces the corresponding fatty acids and glycerol.
Alkaline hydrolysis, saponification, produces glycerol and a mixture of fatty acid salts - soap.
Waxes[edit | edit source]
Waxes are esters of higher fatty acids and higher monohydric alcohols. Examples can be cetyl alcohol with sixteen carbon atoms, ceryl alcohol with twenty-six carbon atoms or myricyl alcohol with thirty carbon atoms.
Complex lipids[edit | edit source]
Complex lipids form the basic building block of cell membranes. It includes phospholipids, glycolipids and lipoproteins. In addition to the hydrophobic part, complex lipids also contain hydrophilic components. They are therefore referred to as polar lipids and form micelles and bilayers.
Phospholipids[edit | edit source]
Phospholipids contain a residue of phosphoric acid H3PO4 in their molecule. Phospholipids include glycerol phospholipids and sphingophospholipids.
Glycerolphospholipids (phosphoacylglycerols)[edit | edit source]
The basis of glycerolphospholipids is a molecule of phosphatidic acid, which consists of glycerol esterified with two molecules of fatty acids and one molecule of H3PO4. Another component can bind to the phosphate group of phosphatidic acid, e.g. choline, serine, ethanolamine, etc. The most numerous group of glycerolphospholipids are phosphatidylcholines (lecithins) and are used as part of biological membranes.
Sphingophospholipids[edit | edit source]
Sphingophospholipids contain the alcohol sphingosine to which other components bind.
- Sphingosine with an attached fatty acid is called a ceramide.
- The most important subgroup are sphingomyelins formed by ceramide with a bound phosphoric acid residue and choline. Sphingomyelins are found, for example, in nervous tissue.
Glycolipids[edit | edit source]
Glycolipids contain one or more monosaccharides. These monosaccharides are glycosidically bound to the lipid part of the mono- or diacylglycerol or sphingosine molecule. Glycolipids include cerebrosides and gangliosides.
- Cerebrosides are formed by a ceramide molecule with bound galactose. They occur mainly in the white matter of the CNS. H2SO4 can bind to cerebrosides. In that case, we call them sulfatides.
- Gangliosides are formed from ceramide to which an oligosaccharide, usually galactose and glucose, is attached. They are found in the ganglia of nerve cells and the gray matter of the CNS.
Lipoproteins[edit | edit source]
Lipoproteins are made up of a combination of lipids and proteins.
.
Essential fatty acids[edit | edit source]
- The human organism can desaturate a fatty acid molecule at most at the 9th carbon position.
- If the double bond is located further away, the body cannot create it, and we must ingest such fatty acids in food and call them essential fatty acids.
Linoleic acid[edit | edit source]
- Linoleic acid is made up of eighteen carbons and two double bonds. The cis double bond is in positions 9 and 12 and is therefore designated as ω-6.
- It is mainly found in vegetable oils, for example sunflower. It has pro-inflammatory effects, like other ω-6 fatty acids, and increases plasma levels of some lipids.
- Linoleic acid is used in the biosynthesis of arachidonic acid.
Arachidonic acid[edit | edit source]
- Arachidonic acid is made up of twenty carbons and four double bonds, it is also an ω-6 fatty acid. It functions as an important precursor of biologically active substances - eicosanoids (prostaglandins, prostacyclins, leukotrienes and thromboxanes).
α-linoleic acid[edit | edit source]
Alpha-linolenic acid consists of eighteen carbons and three double bonds. The cis double bond is located in positions 9, 12 and 15, therefore it is designated as ω-3. It is mainly found in fish and marine animals. It lowers the level of cholesterol and TAG in the body and thus reduces the risk of cardiovascular diseases, it also has anti-inflammatory effects.
The importance of lipids for the body[edit | edit source]
- Lipids are the most reduced and therefore the most energy-rich nutrient. They are thus used as an important energy substrate. However, some tissues, such as the brain, cannot normally use them in their metabolism. Due to their hydrophobicity, which means that they do not bind water, unlike carbohydrates, they are the most efficient energy storage.
- Other functions
- Structural functions – for example, they form a necessary component of all cell membranes.
- Mechanical and protective function – subcutaneous fat and fat around organs isolates thermally and physically, myelin sheaths isolates neurons electrically.
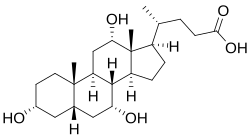
- Important solvents of some substances (fat-soluble vitamins) and are the starting material for the synthesis of many substances important for the body - eicosanoids, steroid hormones, bile acids, etc.
Isoprenoids[edit | edit source]
- In the organism there is a group of substances whose structure is derived from the isoprene molecule - they are made up of two or more isoprene units.
- Steroids, derivatives of triterpenoids, contain six isoprene units. The most important representative of steroids, cholesterol, forms part of cell membranes, and its derivatives (bile acids, steroid hormones) perform many important functions in the body.
Nutrient overview - Proteins[edit | edit source]
- Proteiny jsou makromolekulární organické látky tvořené řetězcem aminokyselin vzájemně spojených peptidovou vazbou. Ta spojuje jednoduchou kovalentní vazbou aminoskupinu jedné aminokyseliny a karboxylovou skupinu druhé aminokyseliny. Polykondenzací vzniká různě dlouhý řetězec aminokyselin ukončený na jedné straně volnou aminoskupinou, N-konec, a na straně opačné volnou karboxylovou skupinou, C-konec.
- Proteins are macromolecular organic substances formed by a chain of amino acids connected to each other by peptide bonds. It connects the amino group of one amino acid and the carboxyl group of the other amino acid by a simple covalent bond. Polycondensation results in a chain of amino acids of varying lengths terminated on one side by a free amino group, the N-end, and on the opposite side by a free carboxyl group, the C-end.
- Peptides are shorter chains of amino acids than proteins (less than 100) and have a molecular weight of less than 10,000.
Protein classification[edit | edit source]
- Proteins can be divided into two basic groups according to their composition.
Simple proteins[edit | edit source]
Simple proteins contain chains made up of only amino acids. We distinguish:
- Fibrillar proteins - scleroproteins
- Fibrillar proteins, insoluble in water, mainly perform structural functions. Individual peptide chains are interconnected by crosslinks so that they form parallel fibers. Examples of fibrillar proteins are collagen or keratin, found in hair, skin or nails.
- Globular proteins - spheroproteins
- The chain of globular proteins has a spherical shape allowing the hydrophobic parts of the molecule to be wrapped inside. They are therefore soluble in water, e.g. albumin.
Complex proteins[edit | edit source]
Složené proteiny obsahují kromě proteinové části i jinou neproteinovou strukturu. Rozlišujeme:
- Glycoproteins – containing a glycosidically linked carbohydrate;
- Metalloproteins – obsahující iont kovu (Fe, Cu), například ferritin nebo transferrin;
- Chromoproteiny – containing a pigment as a prosthetic group, for example hemoglobin, cytochromes or myoglobin;
- Nucleoproteins – containing linked nucleic acids;
- Lipoproteins – containing lipids.
Protein structure[edit | edit source]
We can distinguish several structures in the protein molecule:
Primary structure[edit | edit source]
- The primary structure is determined by the sequence of amino acids in the protein chain. We read their order from N- to C-end. The amino acids of the chain are connected to each other by peptide bonds.
Secondary structure[edit | edit source]
- The secondary structure is conditioned by the formation of hydrogen bonds between the NH– and C=O groups of the peptide bond. The most common secondary structures include the alpha-helix and the beta-folded sheet.
- Alpha-helix
- The protein chain is coiled into a right-rotating or left-rotating helix with a single loop length of 3.6 amino acid residues. The side chains protrude outward from the helix.
- Beta sheet
- There are two chains, arranged in parallel and antiparallel. They stabilize each other by H-bridges.
- In addition to these two secondary structures, there are many others, such as Zn-finger or Leu-zipper.
Tertiary structure[edit | edit source]
- Tertiary structure describes the spatial arrangement of a molecule conditioned by interactions between side groups of the chain – electrostatic forces, H-bridges, SH-bonds, non-polar interactions, etc.
Quaternary structure[edit | edit source]
- Quaternary structure describes the spatial arrangement of protein subunits composed of more than one chain. The subunits are not linked to each other by peptide bonds.
- Denaturation is a process in which all higher protein structures except the primary one are disrupted. The protein loses its functionality, but its energy value remains. Causes may include high temperature, pH change, or the presence of heavy metal salts.
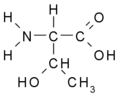
Amino acids[edit | edit source]
Aminokyseliny jsou základní stavební složkou proteinů a peptidů. Obsahují nejméně jednu amino- (–NH2) a jednu karboxylovou (–COOH) skupinu.
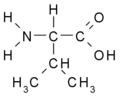
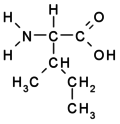
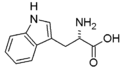
Amino acids are the basic building blocks of proteins and peptides. They contain at least one amino- (–NH2) and one carboxyl (–COOH) group.
According to biosynthesis, we can divide them into:
- Non-essential that the body can synthesize: Gly, Ala, Pro, Ser, Tyr, Cys, Asp, Asn, Glu, Gln
- Essential, which we must ingest in food
- Branched: Val, Leu, Ile
- aromatic: Phe, Trp
- basic: His and Arg (both essential only in childhood or in critical conditions), Lys
- with sulfur content: Met
- with the OH− group: Thr
The importance of proteins for the body[edit | edit source]
The importance of proteins for the human body is enormous. Furthermore, they represent the only source of nitrogen for the body.
Functions:
- structural – collagen, elastin;
- motor - actin, myosin;
- informational - protein hormones;
- defensive – immunoglobulins, complement, antigens;
- transport – albumin, enable catalysis (enzymes) and others.