Nutrient chemistry
Content of the subsection
- Nutrient overview – sacharides
- Nutrient overview – lipids
- Nutrient overview – proteins
Nutrient overview - sacharides
Clasification and structure
Sacharides, also called carbohydrates or glycids, are the most abundant organic substances on Earth. Their molecules are made up of oxygen, carbon and hydrogen atoms. From a chemical point of view, these are polyhydroxyaldehydes and polyhydroxyketones. They contain functional aldehyde or keto groups in their molecule, as well as a larger number of hydroxyl groups.
Clasification of carbohydrates
According to the number of units in the molecule, we distinguish:
- monosaccharides – cannot be further hydrolyzed into simpler units;
- oligosaccharides – they form 2–10 units of monosaccharides by hydrolysis;
- polysaccharides – hydrolyzing into more than 10 monosaccharides.
- Monosaccharides and oligosaccharides are generally called sugars. A synonym for polysaccharide is the word glycan.
We divide monosaccharides according to:
- Number of C-atoms: trioses, tetroses, pentoses, hexoses.
- Functional groups: aldoses and ketoses.
We divide polysaccharides into:
- Homopolysaccharides: these are polymers made up of the same type of monosaccharide. Examples are starch, glycogen or cellulose.
- Heteropolysaccharides: they are polymers made up of more than one type of monosaccharide. An example is hemicellulose.
Structure of saccharides
The structure of a saccharide molecule can be expressed in different formulas:
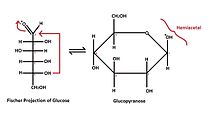
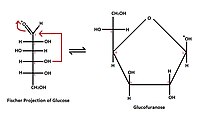
- Linear (Fischer) formula;
- Cyclic (Haworth) formula, which results from the formation of a heterocyclic structure.
- The cycle can contain:
- six atoms - pyranose - similar to six-carbon pyran;
- five atoms - furanose - similar to five-carbon furan.
- Tollens' formula describes the creation of a cyclic structure from a linear formula. It shows the reaction of hydroxyl with a carbonyl group to form a so-called hemiacetal structure.
Isomerism
It is a state where compounds with the same general formula have a different structural arrangement of atoms in the molecule. The following types of isomerism are found in carbohydrate molecules.
- D- and L- prefixes
- It is determined by the position of the −OH group on the last chiral carbon. Assignment of the prefix is based on similarity with the original compound of the carbohydrate series – glyceraldehyde. The −OH group is located on the right for D- and on the left for L- isomers in the Fischer formula.
- D- and L- isomers are mirror images - so-called enantiomers - optical isomers. They differ in the sign of optical rotation, or the direction in which they rotate the plane of polarized light.
- However, it is not generally the case that the D-isomers rotate light to the right and the L-isomers rotate light to the left.
- An equimolar mixture of enantiomers is called a racemic mixture, or a DL mixture, and does not exhibit optical activity.
- D-isomers are more common in nature.
- Pyranoses and furanoses
- They are labeled according to the similarity of the cyclic form of the respective monosaccharide with the pyran or furan cycle. Glucose in solution occurs more than 99% in the form of gluco-pyranose, the rest of the molecules, less than 1%, then appears in the form of gluco-furanose.
- α- a β- anomers
- They are labeled according to the position of the hemiacetal or hemiketal −OH in the cycle. Hemiacetals are formed by the reaction of aldehyde and alcohol groups, hemiketals by the reaction of keto and alcohol groups.
- If the −OH group is oriented to the same side as the −OH group indicating belonging to the D- or L- group, it is an α-anomer. If the −OH group is oriented to the opposite side, it is a β-anomer.
Anomers differ in optical rotation.
- Epimers
- hey differ from each other by the position of one −OH group in the molecule.
Examples are glucose and mannose.
- Aldoses a ketoses
- They are labeled according to the functional group on the 1st and 2nd carbons of the molecule.
Carbohydrates are not essential for the body and are normally synthesized in it, e.g. from amino acids or glycerol.
- Monosaccharides and disaccharides represent an important source of energy. They are especially necessary for brain cells and erythrocytes.
- Polysaccharides serve as energy storage - glycogen in animals.
Carbohydrates also perform structural functions, for example as part of glycoproteins and glycolipids in membranes.
Iron
They also play a key role in the synthesis of nucleic acids or coenzymes. They are also part of the intercellular mass, for example in proteoglycan molecules.