Addison's disease
|
This article was marked by its author as Under construction, but the last edit is older than 30 days. If you want to edit this page, please try to contact its author first (you fill find him in the history). Watch the discussion as well. If the author will not continue in work, remove the template Last update: Wednesday, 08 Nov 2023 at 10.44 pm. |
Addison's disease is associated with a disorder of the adrenal cortex, when the production of the relevant hormones - cortisol and aldosterone - is reduced . It is estimated that 80–90% of the adrenal cortex tissue must be destroyed for symptoms of adrenocortical insufficiency to manifest.
Basic information[edit | edit source]
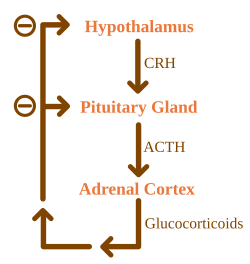
Basic diagram of cortisol regulation[edit | edit source]
Addison's (adrenal insufficiency/panhypocorticalism/hypocortisolism):
The hypothalamus forms the compound CRH (corticotropin releasing hormone). It acts on the pituitary gland, which starts to produce more of the hormone ACTH (adrenocorticotropic hormone). ACTH travels through the blood to the adrenal glands, where it increases the production of cortisol. Glucocorticoids, of which cortisol is the main hormone, have a great influence on the body's metabolism and immunity, enabling a person to survive highly stressful situations.
Basic diagram of aldosterone regulation[edit | edit source]
The kidneys produce the hormone renin, which activates angiotensinogen to angiotensin I. Angiotensin I is converted by ACE (angiotensin converting enzyme) into angiotensin II, which is already biologically active and stimulates the production of aldosterone.
Mineralocorticoids, the main representative of which is aldosterone, have an effect on the kidneys and blood vessels - they reduce the loss of sodium in the urine, increase the loss of potassium and increase blood pressure.
Epidemiology[edit | edit source]
The incidence is approximately 5 cases per 100,000 population/year.
Causes[edit | edit source]
According to the causes, we distinguish 2 forms:
- Central form of Addison's disease ('white Addison') = Hypothalamic and/or pituitary damage - less common. The cause is tumors , injuries and infections of the brain . The adrenal cortex is completely fine (it just lacks the controlling hormone), aldosterone production is usually preserved (aldosterone is controlled by renin).
- Peripheral form of Addison's disease = Damage to the adrenal cortex - It is more common. The cause of the formation of antibodies against one's own cells is unknown, and genetics and some viral infection, which is related to the targeting of the immune system against one's own cells, are probably involved . However, the cortex of the adrenal glands can also be suddenly destroyed during certain infections , classically during meningococcal. infection . This microbe sometimes causes such a severe infection that both adrenal glands bleed and are completely destroyed, which is often associated with the death of the patient. This bleeding of the adrenal glands in meningococcal infections is called Waterhouse–Friderichsen syndrome.
Pathogenesis[edit | edit source]
The cause may be primary adrenocortical insufficiency, i.e. adrenal gland failure, or, more rarely, secondary adrenocortical insufficiency, i.e. lack of ACTH. About 80% [1] of primary diseases are caused by autoimmune damage to the adrenal glands. Other causes include adrenal tuberculosis (about 20% of cases) and a number of rare diseases (adrenal bleeding - Waterhous-Fridrichsen syndrome ; adrenal vein thrombosis; damage to the adrenal glands by infection; destruction of the adrenal glands by tumor metastases, amyloid, sarcoidosis, hemochromatosis; adrenoleukodystrophy; congenital adrenal hypoplasia; ACTH resistance syndromes). Secondary disorders are related to various types of damage to the hypothalamo-pituitary pathway, e.g. tumor, meningitis, hemorrhage, ischemia, etc.
[edit | edit source]
Genetically predisposed individuals have autoantibodies against 21-hydroxylase and lose the ability to produce cortisol over time. These antibodies are present in more than 90% of patients with recent onset of the disease. This susceptibility to the production of autoantibodies is caused by genes encoding MHC type II (mostly found in haplotypeDR4, DR3/4, DQ2/DQ8, DRB1∗0404 subtype DR4). A 5.1 molecule polymorphism (associated with MHC type I) is also associated with a higher probability of developing Addison's disease. This molecule is responsible for the maturation of T cells in the thymus.
Manifestations of the disease[edit | edit source]
A long-term deficiency of adrenal cortex hormones is manifested by general fatigue, low blood pressure, weight loss, occasional abdominal pain and thirst. The patient also has a greater appetite for highly salted foods. A decrease in aldosterone concentration leads to higher sodium losses and potassium accumulation in the body. A high value of potassium leads to diarrhea and can even cause fatal heart rhythm disorders.
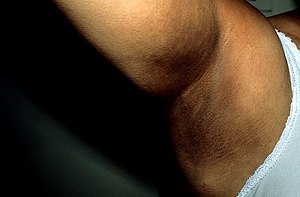
When the adrenal cortex is damaged, the pituitary gland and hypothalamus recognize the low concentration of cortisol in the body and try to increase its concentration - they start producing a lot of CRH and ACTH. This is related to the development of hyperpigmentation of the skin and mucous membranes. The precursor of ACTH is POMC (proopiomelanocortin), from which MSH (melanocyte-stimulating hormone) is also produced. As the name suggests, MSH activates melanocytes , which begin to produce an increased amount of pigment ( melanin ). For this reason, the patient paradoxically looks very healthy and tanned (even in the winter months), and dark graphite spots form on the mucous membrane of the oral cavity .
In the central form of Addison's disease, the production of CRH and ACTH is reduced. Therefore, the substance causing pigmentation is not increased, and "tanned" skin and graphite spots do not appear in this case. In addition, in the case of the central form, the production of aldosterone is not impaired, so the symptoms of the disease will be somewhat milder.
Addison's disease can be divided into 3 clinical forms :
- Part of autoimmune polyendocrine syndrome type 1 ( APS-1 ).
- Part of APS-2 .
- Isolated form.
If there is a critical lack of glucocorticoids and mineralocorticoids in the body, an acute adrenocortical (also Addisonian) crisis occurs . Clinical symptoms include weakness, apathy, confusion, high fever. In the blood there is hyperkalemia, hyponatremia, hypoglycemia, neutropenia, lymphocytosis, monocytosis, eosinophilia. Due to loss of appetite and vomiting, fluid loss, acute hypovolemia and arterial hypotension may occur , resulting in circulatory shock.
Diagnostics[edit | edit source]
Clinical symptoms (weakness, fatigue, anorexia, myalgia, nausea, "craving" for salt, orthostatic hypotension), lower sodium concentration and higher potassium concentration in the blood lead to the doctor's diagnosis . The diagnosis is confirmed by evaluating the concentration of ACTH and cortisol in the blood. Cortisol will always be reduced in Addison's disease. ACTH will be reduced only in the central form of Addison's disease (ie, hypothalamic and/or pituitary dysfunction). ACTH will be elevated in an adrenal cortex disorder.
Morning fasting serum cortisol values below 150 nmol/l confirm the diagnosis of adrenocortical insufficiency, values above 550 nmol/l exclude this diagnosis. Between these values lies a gray zone where patients may have partial insufficiency. We detect such patients using dynamic stimulation tests , which evaluate the ability to increase cortisol secretion under stressful conditions. An insulin tolerance test or synacthen test is used . Insulin tolerance test consists in inducing hypoglycemia (below 2.2 mmol/l) by administering insulin and repeated measurements of the plasma cortisol level. Physiologically, hypoglycemia causes an increase in cortisol production, with hypocorticalism, the secretion of this hormone increases less or not at all. The synacthen test , in which cortisol production is stimulated by the administration of an ACTH analogue, is considered a safer examination method.
Antibodies against 21-hydroxylase can be found in the blood (in up to 90% of patients) with damage to the adrenal glands caused by autoimmunity . Up to 50% of people affected in this way also have another autoimmune disease (antibodies against the thyroid gland, parathyroid glands, testicles, ovaries, gastric mucosa, etc. can be formed). Screening of patients with type 1A diabetes hypoparathyroidism and 21-hydroxylase antibodies is recommended to prevent acute adrenocortical crisis .
We can examine the state of the adrenal glands with ultrasound (we can find damage, but we do not observe autoimmune inflammation), the state of the hypothalamus and pituitary gland can be evaluated using CT and magnetic resonance imaging.
Therapy[edit | edit source]
Treatment of acute Addisonian crisis[edit | edit source]
Treatment should be started as soon as possible. We administer parenterally hydrocortisone in the first 24 hours in doses of 100 mg every 6 hours (in these doses it also has a mineralocorticoid effect). The improvement of the condition is usually fast, so it is possible to reduce the dosage to 50 mg every 6 hours from the second day. Depending on the patient's condition, we gradually reduce the dosage to 10 mg 4 times a day. If it is Addison's disease, we add fludrocortisone (for a mineralocorticoid effect) when hydrocortisone doses are reduced to 50-60 mg/day. In addition to cortisol, we focus on correcting dehydration, which tends to be significant, and electrolyte imbalance. Hyperkalemia and acidosis usually resolve after administration of cortisol. Treatment of the cause of the crisis (infection) is a matter of course.
Chronic treatment[edit | edit source]
Patients with adrenocortical insufficiency require lifelong replacement therapy. The total daily dose of hydrocortisone is usually in the range of 25-30 mg/day, but can be significantly higher. Unfortunately, we do not have any laboratory test that would show the adequacy of the dose, so we are guided by the clinical picture (fatigue, weight, GIT problems), blood pressure and mineralogram. Cortisol is given twice a day - 2/3 of the dose in the morning and the remaining third at 4-5 p.m. The dose of cortisol must be increased during any mental or physical stress, up to doses of 300-400 mg/day. It should always be remembered that short-term administration of a higher dose is practically without risk, while underdosing can cause a crisis! Patients must be properly instructed about this and seek medical attention in case of temperature above 39 °C, diarrhea or vomiting. In Addison's disease, in addition to hydrocortisone, we usually have to administer fludrocortisone with a mineralocorticoid effect. It is administered once a day at a constant dose of 0.05–0.2 mg.
Prognosis[edit | edit source]
An untreated disease leads to the death of the patient within two years. Adrenocortical insufficiency, adequately treated, has a favorable prognosis with survival close to that of the normal population and good quality of life.
Links[edit | edit source]
[edit | edit source]
External links[edit | edit source]
- Addisonova choroba at stefajir.cz
- Adrenální nedostatečnost on Medicabaze.cz, Author: Prof. MD Michal Kršek CSc.
- National Endocrine and Metabolic Disease Information Service
- Template:Acute Addisonian crisis - interactive algorithm + test
Taken from[edit | edit source]
- ŠTEFÁNEK, Jiří. Medicína, nemoci, studium na 1. LF UK [online]. ©2008. [cit. 31.12.2011]. <https://www.stefajir.cz/?q=addisonova-choroba>.
References[edit | edit source]
- MICHELS, Aaron W – EISENBARTH, George S. Immunologic Endocrine Disorders [online]. ©2010. [cit. 2011-04-01]. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2835296/?tool=pubmed>.
- NEČAS, Emanuel. Patologická fyziologie orgánových systémů. Čast 2. 2. edition. 2009. 760 pp. pp. 574-575. ISBN 978-80-246-1712-1.
- ČEŠKA, Richard. Interna. 1. edition. 2010. 855 pp. pp. 347–350. ISBN 978-80-7387-423-0.
- GREENSPAN, Francsi S – BAXTER, J. D. Základní a klinická endokrinologie. 1. edition. 2003. 843 pp. pp. 354–363. ISBN 80-86022-56-0.