Apoptosis
Apoptosis [1](from the Greek apoptosis – falling), or programmed cell death, is a mechanism used to eliminate unnecessary or damaged cells. It is the death of the cell caused by the activation of cysteine proteases, caspases, and then nuclear endonucleases. Nuclear DNA is damaged and all biosynthetic processes in the cell stop. It plays a great role in ontogenesis, when it helps to form organs.
During apoptosis (unlike necrosis), there is no swelling, bursting, and spilling of cell contents that would cause an inflammatory response.
| Criteria | necrosis | apoptosis |
| Cell size | ↑↑↑ | ↓↓↓ |
| Core | Basophilia disappears, decay | Chromatin condensation |
| Plasma membrane | Rupture | Formation of apoptotic bodies |
| Organelles | Zooming out + swelling | Aggregation + lysis |
| Inflammatory response | yes | No |
| Genetics | (No) | Yes |
| Occurrence | Anywhere | Rapidly dividing tissues of the embryo, involution |
Morphological manifestations[edit | edit source]
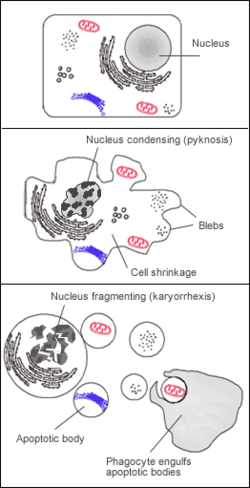
It manifests itself only microscopically. Condensation and fragmentation of the cell leads to the formation of apoptotic bodies (fragments of a decayed cell surrounded by a membrane). It is never massive enough to cause macroscopic changes in the organs.
Basic manifestations of apoptosis
- Pyknotization of the nucleus - puckering and darkening of the nucleus.
- Wrinkling of the whole cell while preserving the integrity of the organelles.
- The cytoplasm becomes darker and parts of it are suffocated as apoptotic bodies. These are phagocytosed by surrounding elements (histiocytes, parenchymal cells).
In phagocytic cells, they can persist for some time as lysosomal apoptotic bodies. E.g. in hepatitis, so-called Councilman's bodies are found in the hepatocytes or Fleming's bodies are found in the centrofollicular macrophages of the lymphatic follicles (apoptosis of functionally defective B-lymphocytes).
Causes and examples of apoptosis[edit | edit source]
- Apoptosis spontaneous (physiological)
Reduction of cell populations in embryogenesis. Disappearance of postnatally changing cells – blood elements, enterocytes, keratinocytes. Elimination of cells in the hyperplastic population. This is a return to the norm after hormonal stimulation, or a reduction of cells with reduced hormonal stimulation (e.g. reduction of the mammary gland after the end of lactation).
- Apoptosis induced by a pathological stimulus
Elimination of cells infected with the TC virus and NK lymphocytes. Numerical atrophy of cells after blockage of gland ducts. Alternatively, it is used in tumor chemotherapy.
Molecular pathways of apoptosis[edit | edit source]
Apoptosis is a process that requires the active participation of the cell in its own demise. It is an energy-intensive event that needs ATP.
The process itself can be divided into two phases – initiator and effector (common).
The initiation phase of apoptosis[edit | edit source]
A variety of factors can be the trigger. Substances that act as ligands of membrane or cytoplasmic receptors, DNA damage, changes in the functional state of mitochondria, lack of growth factors, radiation, etc.
The induction of the process of cell death most often occurs in two ways - internal (intrinsic) and external (extrinsic).
Intrinsic pathway[edit | edit source]
Physiologically, it occurs, for example, during embryogenesis or at the end of hormonal stimulation. Furthermore, the internal - mitochondrial apoptotic pathway can be initiated by a number of changes in the microenvironment of the cell, including DNA damage, defective mitosis, endoplasmic reticulum stress, reactive oxygen species, etc.
The cell contains pro-apoptotic (Bax, Bak) and anti-apoptotic (Bcl-2, Bcl-X) factors that regulate cell death. These factors must be in balance with each other. When they deviate (↑ pro-apoptotic), apoptosis occurs. They occur mainly on the outer membrane of mitochondria.
The action of inhibitory factors (Bim, Bid) on anti-apoptotic factors results in their inactivation. Pro-apoptotic factors begin to predominate, which begin to create channels in the mitochondrial outer membrane permeabilization (MOMP). Cytochrome C escapes through the newly formed channels into the cell cytoplasm, where it binds to APAF1 (apoptotic peptidase activating factor 1). Procaspase 9 then binds to this complex. We call the resulting complex an apoptosome. Its function is the formation of fully active caspase 9, which is important for the initiation of the effector phase (proteolytic activation of executioner caspases 3 and 7).
Extrinsic Pathway[edit | edit source]
A process that is activated through immune cells (e.g. cytotoxic CD8+ T-lymphocytes, NK-cells). Immune cells with their ligands (Fas-ligand) bind to death receptors on the cell membrane (TNF-receptor, Fas-receptor).
These receptors contain a so-called death domain (DD) on the intracellular part. Ligands are expressed only on the surfaces of immune cells, death receptors are part of the membrane of all cells of the organism's tissues.
The death domain (in the case of Fas-L + Fas-R interaction it is FADD – Fas associated death domain) serves to activate caspase 8. Like caspase 9 in the intrinsic pathway, caspase 8 participates in the initiation of the effector phase of apoptosis.
Effector phase of apoptosis[edit | edit source]
There is a convergence of both the previous two pathways[2].
Caspases 8 and 9 activate effector caspases (3, 6, 7). These caspases cleave cellular proteins – actin, lamin, repair proteins, etc. Their action activates endonuclease, which causes genomic DNA fragmentation. After activation of effector caspases, the process is irreversible.
Even after genetic knockout of caspases, apoptosis can occur (apoptotic pathway independent of caspases). One hypothesis describes that high intracellular calcium concentration (such as in severe ATP deficiency) leads to the activation of the calpain protease, which releases apoptosis-inducing factor (AIF) from the inner mitochondrial membrane. AIF diffuses into the cytosol and into the nucleus, where it condenses chromatin and induces apoptosis[3].
Mutations in the genes of these proteins leading to the inhibition of apoptosis are one of the steps in tumor transformation.
Evidence of apoptosis[edit | edit source]
- Histologically in HE (apoptotic bodies), possibly silvering.
- Electron microscopy.
- Histochemically - TUNEL method.
- Electrophoresis – detection of DNA fragments.
- Propidinium iodide – will also show necrotic cells.
- Nexin - in tissue cultures
links[edit | edit source]
[edit | edit source]
- Apoptosis signaling disorders in tumor cells
- Apoptosis and clinical consequences of disorders of its regulation
- Necrosis
- Caspases
- T-lymphocytes
- NK cells
- Genetic causes of the aging process and death
Sources[edit | edit source]
- PASTOR, Jan. Langenbeck's medical web page [online]. ©2004. [cit. 2011]. <https://langenbeck.webs.com/>.
Reference[edit | edit source]
- ↑ ALBERTS, B., D. BRAY a A. JOHNSON. Základy buněčné biologie. 2. vydání. Espero Publishing, 2005. 740 s. s. 584-587. ISBN 80-902906-2-0.
- ↑ LE, Tao, Vikas BHUSHAN a J. TOLLES. First aid for the USMLE Step 1. 21. vydání. McGraw Hill, 2011. 578 s. s. 220. ISBN 0071742301.
- ↑ https://en.wikipedia.org/wiki/Apoptosis-inducing_factor
recommended literature[edit | edit source]
- MASOPUST, Jaroslav a Richard PRŮŠA. Patobiochemie buňky [online] . 1. vydání. Praha : Univerzita Karlova v Praze, 2. lékařská fakulta, 2003. 344 s. Kapitola 2
- Apoptóza. s. 240-257. Dostupné také z <http://mefanet-motol.cuni.cz/clanky.php?aid=218>. ISBN 80-239-1011-6.