Artificial pulmonary ventilation (neonatology)
This article discusses the use of UPV in neonatology. Other articles related to the topic: Introduction to artificial pulmonary ventilation • Artificial pulmonary ventilation • Artificial pulmonary ventilation/SŠ (nurse).
Goals of artificial pulmonary ventilation (UPV):
- adequate oxygenation (blood oxygenation);
- adequate ventilation (excretion of carbon dioxide);
- minimal lung damage (lowest possible pressures, early extubation).
Indication:
- neonatal pneumopathy, heart failure, neuromuscular disease, effects of drugs (e.g. general anesthesia), weakness, airway pathology, …
Options for respiratory support for a newborn:
- administration of oxygen (oxygen therapy) - to the incubator or through nasal cannula with low flow (low flow nasal cannula);
- non-invasive respiratory support – high flow nasal cannula, nasal CPAP;
- conventional ventilation – mandatory or synchronized, pressure- or volume-controlled, hybrid modes;
- unconventional ventilation – high-frequency, jet;
- extracorporeal oxygenation (ECMO).
Monitoring during respiratory support:
- non-invasive: blood oxygen saturation (pulse oximetry), breathing and heart rate, blood pressure, body temperature;
- blood gases;
- normal paO2 range: 7-10 kPa (50-75 mmHg);
- normal range of paCO2: 4.6-5.4 kPa (35-40 mmHg);
- permissive hypercapnia (tolerance of higher paCO2 provided an acceptable pH, i.e. pH > 7.25) reduces the risk of developing bronchopulmonary dysplasia;
- hypocapnia: with each decrease in paCO2 by 1 kPa, cerebral blood flow decreases by about 30%; the risk of developing periventricular leukomalacia increases;[1]
- X-ray of the chest (to assess lung inflation, verify the position of the endotracheal cannula, to diagnose lung pathologies).
Care of the newborn with respiratory support'
- Minimal handling, quiet environment, positioning (changes in position help the movement of secretions in the airways), suctioning from the airways and physiotherapy (only in indicated cases), adequate nutrition.[1]
Oxygen Administration[edit | edit source]
- Oxygen is given warm and humidified. Inhaled oxygen concentration (FiO2 0.21 or 21% corresponds to air inhalation, FiO2 1.0 or 100% corresponds to 100% oxygen inhalation) and blood oxygen saturation are monitored (using pulse oximetry).
- It can be administered in an incubator or through nasal cannulae. The disadvantage of administration to the incubator is the fluctuation of the level of oxygen administered each time the incubator door is opened.
- Used in children with mild symptoms of RDS or TTN. In case of respiratory failure or the need for a high concentration of oxygen, it is necessary to provide adequate respiratory support (e.g. NCPAP or mechanical ventilation).[1]
- Target saturation: ≥ 95%.
- Target saturations of 91-95% are recommended for oxygen administration in preterm infants born before 28 weeks of gestation. In 2010, the results of the SUPPORT study comparing low (85-89%) and high (91-95%) target saturations were published. The group of children with low saturations had a lower incidence of severe retinopathy of prematurity, but higher mortality, so targeting higher saturations is now recommended. However, optimal saturation values are not yet fully understood.[2]
- Oxygen is toxic to tissues due to its ability to form oxygen free radicals such as superoxide (O2−) and hydroxyl (OH− sup>). To defend against these free oxygen radicals, tissues produce antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. Despite this, breathing 100% oxygen is proven to damage the lungs.[1]
High Flow Nasal Cannula (HFNC)[edit | edit source]
- A high flow nasal cannula or oxygen/air mixture (high flow nasal cannula) is used as an alternative to NCPAP.[1]
Nasal CPAP (NCPAP)[edit | edit source]
- CPAP (Continuous positive airway pressure) is positive pressure continuously administered to the airways. With nasal CPAP, CPAP is administered through short tubes inserted into the nostrils. The goal is to keep the alveoli and airways open and prevent them from collapsing during exhalation. When administering CPAP, it is necessary to insert an orogastric tube to decompress the stomach.
- Indications: RDS, post-extubation respiratory support, apnoeic pauses (especially mixed and obstructive), …
- Setting: Newborns with RDS who have not been given surfactant usually need pressures around 5-8 cm H2O;[1] at better lung compliance lower pressures are enough. Pressures that are too high can cause the lungs to overexpand, resulting in impaired ventilation and carbon dioxide retention.
- Monitoring: CPAP and FiO2 are adjusted depending on blood gas analysis. When high CPAP pressures and a high fraction of inhaled oxygen are required, intubation and artificial pulmonary ventilation are necessary.
- Complications: Bruising and damage to the skin of the nose and face occur relatively often; pneumothoraxu may occur.[1]
Neonatal intubation[edit | edit source]
- Intubation is the insertion of an endotracheal (ET) tube into the trachea. Sterile ET cannulas without an inflatable cuff with an inner diameter of 2.5–4 mm are used (for neonates weighing < 1 kg: 2.5 mm; 1–2 kg: 3.0 mm; 2–3 kg: 3.5 mm ; over 3 kg: 4 mm). The ET cannula can be inserted through the mouth or nose. During oral intubation, the ET cannula insertion depth can be derived according to the formula: 6 + the child's weight in kg (ie, for a child weighing 1.5 kg, we fix the ET cannula at 7.5 cm at the level of the lips). In neonates, laryngoscopes with straight spoons of sizes 00 to 1 are used (size 00 for children weighing < 1 kg; size 0 for 1–3 kg; size 1 > 3 kg). An introducer can be used to insert the ET cannula.[3] Successful intubation can be demonstrated by a clinical response (increased heart rate), visible chest movements, auscultation over the lungs, or a capnometer (by measuring exhaled carbon dioxide). Correct insertion position can be verified using a chest x-ray (the end of the ET cannula should be below the vocal cords and above the carina, or approximately at the Th1–Th4 level).
- A distinction is made between urgent (immediate) and elective (planned) intubation.
- Before elective intubation, premedication is recommended to prevent the release of stress hormones accompanied by a rise in blood and intracranial pressure (with the subsequent risk of developing intracranial bleeding).
- A combination is used for premedication:
- atropine (vagolytic effect – prevention of bradycardia caused by e.g. myorelaxant administration);
- analgesia (fentanyl has a faster onset of action than morphine; may cause chest wall stiffness which can be relieved by slow administration and subsequent administration of myorelaxation – suxamethonia);
- myorelaxation (prevention of an increase in intracranial pressure; eg: mivacurium or succinylcholine).[1]
| Baby weight: | < 1 kg | 1-2 kg | 2-3 kg | > 3 kg |
|---|---|---|---|---|
| Laryngoscope spoon size: | 00 | 0 | 0 | 1 |
| Inner diameter of ET cannula: | 2.5 mm | 3.0 mm | 3.5 mm | 3.5-4 mm |
| Insertion depth (from lip): | 6-7 cm | 7-8 cm | 8-9 cm | 9 cm |
Sedation during ventilation[edit | edit source]
Sedation[edit | edit source]
- Morphine is the gold standard for analgesia. In mechanically ventilated neonates, an initial dose (50–150 µg/kg) followed by a continuous infusion (5–20 µg/kg/h) is used. Tolerance develops over time, so it is necessary to increase the dose. Withdrawal syndrome can occur after 48 hours of continuous morphine infusion, but is usually observed only after 4-5 days. Morphine (in decreasing doses) or methadone, as well as clonidine, or benzodiazepines.
- Chloral hydrate (30–50 mg/kg p.o. or p.r.), promethazine (0.5 mg/kg i.v./p.o.) and other drugs.[4]
Paralysis[edit | edit source]
- Indication: mature neonate with meconium aspiration syndrome, persistent pulmonary hypertension or GBS sepsis who is restless, hypoxic or asynchronous with ventilator despite sedation. A newborn with congenital diaphragmatic hernia or severe pulmonary interstitial emphysema. A newborn who is actively exhaling against the ventilator despite sedation.[1]
- Pancuronium in the form of boluses - the need to monitor fluid balance due to the risk of fluid retention;
- Vecuronium.[1]
Conventional artificial lung ventilation[edit | edit source]
Variables of artificial pulmonary ventilation[edit | edit source]
- FiO2 (fraction of inspired oxygen) = oxygen concentration in inspired air (0.21-1.00 or 21-100%);
- PIP (peak inspiratory pressure) = maximum inspiratory pressure; opens the alveoli;
- PEEP (positive end-expiratory pressure) = positive end-expiratory pressure; prevents the collapse of the alveoli during exhalation; maintains functional residual lung capacity;
- MAP (mean airway pressure) = mean pressure in the airways; affects oxygenation; too high MAP reduces venous return and thereby reduces cardiac output;
- MAP = (PIP — PEEP) × [Ti : (Ti + Te)] + PEEP.
- VT (tidal volume) = tidal volume;
- Ti (iT) = inspiratory time, Te (eT) = expiratory time;
- (respiratory) rate = number of breaths per minute; depends on the length of inspiratory and expiratory time (and vice versa).
- compliance of the lungs
- characterized by the compliance (elasticity and extensibility) of the alveoli, chest wall and lung parenchyma – the lower the compliance, the stiffer (less compliant) the lungs;
- Lung compliance is reduced in surfactant deficiency, pulmonary hypertension, ARDS, pneumonia, cardiopulmonary bypass, ...
- with reduced compliance, optimal PEEP, higher PIP and higher inspiratory time are required.
- 'Resistance of the respiratory tract
- airway resistance is increased in bronchospasm or tracheobronchomalacia;
- the endotracheal cannula also contributes to airway resistance – its resistance is inversely proportional to the fourth power of the diameter of the ET cannula (the narrower the ET cannula, the significantly higher the resistance);
- with increased resistance, a lower breathing frequency and a longer exhalation time are needed.
Oxygenation[edit | edit source]
- blood oxygenation - optimally according to the current needs of the organism
- is influenced by hemoglobin binding capacity and cardiac output; and pulmonary and cardiac shunts
- depends on fraction of inspired oxygen (FiO2) and mean airway pressure (MAP)
- MAP depends on PIP, PEEP, iT
- monitored using non-invasive measurement of hemoglobin oxygen saturation, blood gas examination (paO2 – partial pressure of oxygen in arterial blood) and lactate level
- oxygen consumption can be reduced by sedation, paralysis, hypothermia.
Ventilation[edit | edit source]
- excretion of carbon dioxide
- depends on minute ventilation (= tidal volume × respiratory rate)
- tidal volume depends on the difference between PIP and PEEP
- monitored using blood gas tests (paCO2 – partial pressure of carbon dioxide in arterial blood).
- so-called permissive hypercapnia is tolerated - that is, a higher pCO2 under conditions of acceptable pH (usually pH > 7.25), which makes it possible to reduce the risk of lung baro/volume trauma.
Types of Conventional Mechanical Ventilation[edit | edit source]
- controlled ventilation (volume or pressure controlled);
- support ventilation modes (volume or pressure support);
- hybrid ventilation modes (combination of different ventilation modes).
Pressure controlled ventilation, PCV (pressure controlled ventilation)[edit | edit source]
It allows delivery of a set peak inspiratory pressure (PIP) and then passive expiration to atmospheric pressure or a preset positive pressure (PEEP) that prevents alveolar collapse. Tidal volumes depend on lung compliance and resistance.
- the number of breaths, PIP and PEEP is set;
- minute ventilation is monitored.
Volume controlled ventilation, VCV (volume controlled ventilation)[edit | edit source]
It allows delivery of a set tidal volume (VT) and then passive expiration to atmospheric pressure or preset positive pressure (PEEP), which prevents alveolar collapse. Peak inspiratory pressures (PIP) depend on pulmonary compliance and resistance.
- tidal volume, number of breaths (or minute ventilation) and PEEP are set;
- airway pressures are monitored.
Ventilation Modes[edit | edit source]
The names and characteristics of the ventilation modes may vary depending on the manufacturer of the ventilator.
- IPPV mode, intermittent positive pressure ventilation (Intermittent Positive Pressure Ventilation)
- CMV (Continuous Mandatory Ventilation) mode
An unsynchronized ventilation mode that is used in paralyzed or apneic patients. The rate is set higher than the patient's spontaneous breathing rate.
- IMV (Intermittent Mandatory Ventilation) mode
Unsynchronized ventilation mode. The frequency is set lower than the patient's spontaneous breathing rate, so the patient can breathe spontaneously between controlled breaths.
- SIMV (Synchronized Intermittent Mandatory Ventilation) mode
Synchronized ventilation mode that supports the set number of breaths per minute. The patient's inspiratory effort (trigger) starts the controlled breath. If the patient is breathing faster, the ventilator will only support the set number of breaths. If the patient breathes slower than the set number of breaths, the ventilator synchronizes all breaths and additionally delivers the necessary number of breaths to reach the set number. PIP, PEEP, respiratory rate, inspiratory time and trigger (sensitivity of inspiratory effort) are set.
- SIPPV (Synchronized Intermittent Positive Pressure Ventilation) or AC (Assist Control) mode
Synchronized ventilation mode to support each inspiratory effort of the patient. Inspiratory support is terminated after the set inspiratory time has elapsed. If the patient breathes slower than the set number of breaths, the ventilator synchronizes all breaths and additionally delivers the necessary number of breaths to reach the set number. If the patient breathes faster than the set number of breaths, all breaths are supported and with a very high breathing frequency or a long inspiratory time there is a risk of air trapping in the lungs and therefore a risk of air leak. PIP, PEEP, respiratory rate, inspiratory time and trigger (sensitivity of inspiratory effort) are set.
- PSV mode, Pressure Support Ventilation (Pressure Support Ventilation)
Synchronized ventilation mode to support each inspiratory effort of the patient (similar to SIPPV). Inspiratory support is terminated when the flow rate drops to the set value. Inspiratory time is variable according to lung filling (inflation). The risk of air trapping and air leak is lower than with SIPPV mode. PIP, PEEP, respiratory rate and termination sensitivity (percentage of maximal inspiratory flow) are set.
- VG (Volume Guarantee) mode
The ventilator delivers a set tidal volume (usually 4-7 ml/kg). The ventilator measures the exhaled volume and accordingly delivers the necessary PIP to achieve the set tidal volume. The maximum PIP is set. If the set tidal volume is not changed at the set maximum PIP, an alarm will be triggered. This mode is usually used in combination with PSV or SIPPV. Does not work with high leakage.
High Frequency Ventilation (HFV)[edit | edit source]
Principle: exchange of very small respiratory volumes at high frequency.
- tidal volumes are comparable to or less than dead space
- frequency is expressed in Hz (1 Hz = 1 cycle/s = 60 breaths/min.)
Advantages: the use of small breathing volumes makes it possible to reduce the risk of barotrauma.
Indications for HFV:
- pulmonary interstitial emphysema (PIE)
- air leak syndromes (pneumothorax, pulmonary interstitial emphysema)
- neonatal respiratory distress syndrome (RDS)
- pre-stage of extracorporeal support (PPHN, MAS, pneumonia, hypoplastic lung, congenital diaphragmatic hernia)[5]
High frequency positive pressure ventilation (HFPPV)[edit | edit source]
- (respiration rate 60–100/min, volume: 3–4 ml/kg)
High Frequency Oscillation Ventilation (HFOV)[edit | edit source]
- uses permanent distension pressure (MAP, mean airway pressure) with very rapid pressure oscillation around MAP; this creates very small tidal volumes, often smaller than dead space
- indication:
- failure of conventional ventilation in a full-term newborn (PPHN, MAS)
- air leak syndromes (pneumothorax, pulmonary interstitial emphysema)
- failure of conventional ventilation in premature newborns (severe respiratory distress syndrome of newborns, pulmonary interstitial emphysema, pulmonary hypoplasia) or to reduce barotrauma when high pressures are needed during conventional ventilation
Variables:
- frequency (Hz): 10 Hz = 10 cycles/s = 600 cycles/min.
- MAP, mean airway pressure (cm H2O) = mean pressure in the airways
- high MAP can reduce cardiac output by reducing venous return and thereby lowering blood pressure
- amplitude = delta P = fluctuation around MAP[6]
High Frequency Jet Ventilation (HFJV)[edit | edit source]
- high speed gas injection;
- frequency 240-600 breaths/min.;
- tidal volumes comparable to or slightly greater than dead space;
- exhalation is passive;
- indication: mainly pulmonary interstitial emphysema (PIE), otherwise see HFV.[5]
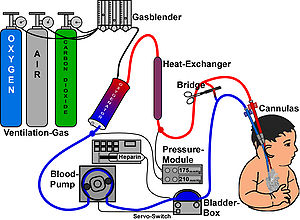
ECMO[edit | edit source]
Complications and negative effects of artificial lung ventilation[edit | edit source]
Complications of intubation[edit | edit source]
- injury, insertion of the endotracheal tube too deep - intubation into the right bronchus, pneumothorax, atelectase.
Lung injury due to artificial lung ventilation[edit | edit source]
- 'Ventilator-induced lung injury (VILI) is an acute injury to the airways and lung parenchyma caused by artificial lung ventilation;
- pathophysiology of VILI: lung damage (cellular and structural damage, alveolar edema) → inflammation → fibrotization and healing → disappearance of edema (reabsorption) → repairs (removal of intraalveolar debris, restoration of the extracellular matrix, re-epithelialization of the alveolar surface, formation of new capillaries);
- barotrauma: rupture of alveoli due to increased transalveolar pressure (high PIP); air leak syndromes (pneumothorax, pneumomediastinum, subcutaneous emphysema)
- volu(mo)trauma: damage by excessive lung inflation (high PEEP+VT);
- atelectotrauma: damage by alveolar collapse with subsequent re-expansion (ventilation without PEEP or without surfactant);
- biotrauma': induction of inflammation (activation of macrophages → release of cytokines TNF-α and IL-1 → stimulation of vascular endothelium to release intercellular adhesion molecule -1 (ICAM-1) and E-selectin; adhesion of neutrophils to the endothelium and transmigration into the interstitial alveolar space;
- ergotrauma: damage by dynamic parameters of ventilation;
- dynamic stress (volume difference, pressure difference, high flow) is more harmful than static stress (PEEP);
- ventilator-associated lung injury (VALI) - the term is used when it is unclear whether there was a worsening of the lung disease itself or whether it was due to artificial lung ventilation (causality is not clear).[7][8]
- auto-PEEP (intrinsic positive end expiratory pressure) - occurs if there is insufficient exhalation, PEEP gradually increases, the risk of barotrauma increases, the patient's ability to trigger inspiration deteriorates,
- heterogeneous ventilation - ventilation of different lung areas is different depending on alveolar compliance, airway resistance and dependence (upper vs. lower lung areas);
- ventilation/perfusion mismatch (increased dead space - areas relatively overventilated compared to perfusion; reduced shunts - areas relatively underventilated compared to perfusion);
- diaphragm muscle atrophy, respiratory muscle weakness,
- reduced mucociliary motility - leads to retention of secretions and development of pneumonia.[9]
Systemic complications of artificial lung ventilation[edit | edit source]
- reduced cardiac output, disruption of hemodynamic monitoring,
- impaired perfusion of the splanchnic, stress ulcers of the digestive tract, hypomotility of the digestive tract,
- fluid retention,
- acute renal failure,
- increased intracranial pressure,
- inflammation,
- disturbed sleep.[9]
Complications of oxygen therapy[edit | edit source]
- oxygen toxicity is caused by reactive oxygen species that can damage tissues by inducing necrosis or apoptosis if the amount of reactive oxygen species exceeds the body's antioxidant capacity.
Links[edit | edit source]
Related Articles[edit | edit source]
- Artificial pulmonary ventilation • Artificial pulmonary ventilation/SŠ (nurse)
- Oxygen Therapy • Hyperbaric Oxygen Therapy • Oxygen Toxicity
References[edit | edit source]
- ↑ a b c d e f g h i j Rennie and Robertson, Section 5 - Chapter 27 - Part 2
- ↑ Rennie and Robertson, Section 5 - Chapter 19
- ↑ a b Rennie and Robertson, Section 5 - Chapter 13
- ↑ Rennie and Robertson, Section 5 - Chapter 25
- ↑ a b GOMELLA, TL, et al. Neonatology : Management, Procedures, On-Call Problems, Diseases, and Drugs. 7. edition. Lange, 2013. pp. 71-89. ISBN 978-0-07-176801-6.
- ↑ http://www.adhb.govt.nz/newborn/guidelines/respiratory/hfov/hfov.htm
- ↑ https://www .uptodate.com/contents/ventilator-induced-lung-injury
- ↑ ATTAR, Mohammad Ali – DONN, Steven M. Mechanisms of ventilator-induced lung injury in premature infants. Seminars in Neonatology. 2002, y. 5, p. 353-360, ISSN 1084-2756. DOI: 10.1053/siny.2002.0129.
- ↑ a b
„ {{{1}}} “