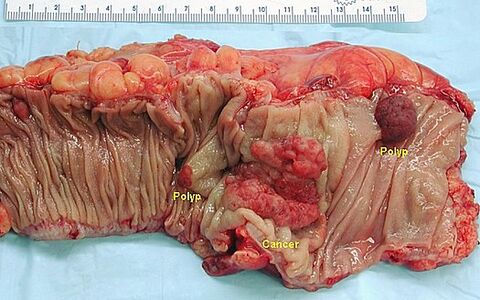
Colorectal carcinoma (PGS)
Colorectal cancer (CRC) is one of the most common cancers in developed countries. About a third of patients die from cancer, but it is still one of the most common causes of cancer-related deaths worldwide. It can arise on the basis of polyps, a smaller number of tumors can arise even without previous polyps. It is stated that a maximum of 85% of colorectal cancer cases occur sporadically under the influence of risk factors, especially lifestyle, the rest of the tumors are of hereditary origin. The actual proportion of hereditary influences is probably much higher, eg milder forms of Lynch syndrome often escape diagnosis. The basis of therapy is surgery, the basis of therapeutic success is early diagnosis, usually through screening when the tumor is clinically silent. Colorectal cancer can occur in several ways, the most common, originally considered to be the only one, is the APC gene mutation path followed by an alteration of the wnt signaling cascade.
History[edit | edit source]
There is no reliable information that colorectal cancer occurs in the prehistoric period. However, this cannot be considered as evidence that colorectal cancer or other tumors do not occur. In fact, soft tissues are preserved as a source for research in the field of historical anthropology or even paleontology only very rarely. Even metastatic bones are more prone to faster decomposition, so preserved traces of metastatic skeletal involvement are relatively rare.[1]
Sources from historical periods are greatly complicated by the fact that the basic tool of physicians of various historical epochs was an external description. Cancer in the modern sense of the word was not usually defined, it could be classified as swelling together with abscesses or between ulcerations together with some infections. From antiquity to the Middle Ages, Galen's theory persisted that the tumor was a local urban of black bile.[1] Only the work of A. Vesalia (1514-1564) and G. B. Morgagni (1681-1771) with a thorough description of tumors questioned the humoral theory and created space for new speculative theories, such as Paracelsus's (1493-1541) theory of mineral occupation from environment or Le Dran's (1685-1770) theory of the lymphogenic origin and spread of tumors. Uncertainties about the origin of tumors have been linked to a number of speculative treatments. The speculative theories about the nature of tumors have not yet prevented modern physicians from using relatively vigorous and dangerous procedures consisting, for example, in the application of toxic substances, such as mercury. In response to the results of such therapeutic efforts, the opposite extremes of the administration of biologically practically inert substances [2] have, of course, also gained popularity. Even in spite of mostly unsuccessful interventions, there have sometimes been relatively sharp conflicts of opinion between supporters of various theories and therapeutic approaches, including public promotion of their approach and attacks on the other side. The clash of proponents of (not only) venereal mercury therapy and proponents of herbal juice and extract therapy is relatively well known. [2][3]
The discovery that tumors are cellular is associated with the work of J. P. Müller (1801 - 28 April 1858) and Th. Schwann (1810-1882) from the end of the first half of the 19th century. The theory describing the terms used today, initiation, growth, local spread and metastatic spread, formulated in 1865 by C. Thiersch (1822-1895), H. W. G. von Waldayer-Hartz (1836-1921), supported this therapy with strong evidence.[2]
The oldest probable description of colorectal cancer in medieval Europe appears in 9th-century Irish texts and 10th-century Saxon texts. The description apparently corresponds to a tumorous obstruction of the large intestine.[2]
One of the oldest surviving descriptions from medieval Europe, which certainly captures colorectal cancer, including a description of the tumor itself and its manifestations and prognosis, was left by the English surgeon John of Arden in the 14th century. Among other things, the author mentions that he saw many people who died of the disease. However, he had never seen or cured anyone of the disease.[2]
The first therapy with the hope of therapeutic success was a radical surgical resection of the affected section of the intestine. The first successful resection of the rectal tumor was performed in 1829 by J. Lisfranc (1790-1847). Rectal cancer surgery became more widespread at the beginning of the 20th century, mainly due to a significant reduction in postoperative mortality. Even in a small proportion of patients who underwent the procedure, about 90% of patients relapsed. With the gradual improvement of technique and scope of performance, perioperative mortality was already 6% in the 1930s and five-year survival increased to 65%.[2] In 1954, W. H. Cole published a work demonstrating that tumor cells may appear in the portal blood of a operated patient during surgery; this finding gradually led to the introduction of the "no touch" concept in colon and rectal surgery.[4] Work safely demonstrating that extending performance to mesorectal fat improves patient survival was not published until R. J. Heald and J. D Ryall.[2]
The discovery of both X-rays and radioactivity, and in particular the discovery that ionizing radiation inhibits cell growth, led to the use of these modalities as supportive therapies as early as the beginning of the 20th century. However, the original methods of application did not significantly affect the survival of patients.[2]
The discovery that mustard gas can inhibit hematological malignancies has also led to attempts to treat colorectal cancer with mustard gas-derived substances (the first alkylating cytostatics). The results of these experiments were not encouraging, colorectal cancer is only slightly sensitive to the effects of alkylating cytostatics. In 1957, fluorouracil was introduced as a representative of a new class of cytostatics (antimetabolites), which proved to be very effective in the treatment of colorectal cancer.[2]
Epidemiology[edit | edit source]

Spread[edit | edit source]
It is estimated that more than one million new patients worldwide appear each year, with approximately one third of patients dying from the disease.[5] According to analyzes at the turn of the 19th and 20th centuries, untreated colorectal cancer ends in death within an average of about two years from the onset of symptoms, with more than 60% of patients dying within two years without anticancer treatment except palliative care; exceptionally, the patient's survival has been observed for many years.[6][7] In comparison with the current results, it can be clearly stated that the prognosis of treated patients is significantly better than patients without cancer therapy.
Together with Slovakia, Hungary, Israel and New Zealand, the Czech Republic is one of the countries with the highest incidence of colorectal cancer.[8]
According to GLOBOSCAN 2012, the age-standardized incidence and mortality are[9]
| sex | age-standardized incidention (per 100 thousand) | age-standardized mortality |
|---|---|---|
| male | 54,0 | 22,6 |
| female | 27,1 | 9,9 |
| both sexes | 38,9 | 15,4 |
These data refer to an age-standardized population, allowing international comparisons between populations with different age structures. The actual incidence of colorectal cancers in the Czech Republic in 2011 was 38.36 cases per 100 thousand, mortality 16.46 per 100 thousand residents. [10] Although the incidence is on the rise, mortality is relatively stable over the long term. The proportion of individual clinical stages does not change much over time, so stable mortality with increasing incidence is more indicative of increasing availability and quality of medical care. [8]
Risk factors[edit | edit source]
Most cases of colorectal cancer occur sporadically. The main risk factors are:[5]
- Demographic factors:
- age,
- male gender.
- Environmental factors:
- red meat intake,
- high fat diet
- sedentary lifestyle,
- smoking,
- alcohol.
- History of diseases:
- history of colorectal polyps,
- history of colorectal cancer,
- diabetes mellitus,
- idiopathic intestinal inflammation.
Genetic syndromes[edit | edit source]
Some genetic syndromes may be associated with a greater or lower risk of developing colorectal cancer. The age of the manifestation may vary. While, for example, on the basis of familial adenomatous polyposis, colorectal cancer develops at a young age, milder forms of Lynch syndrome can lead to colorectal cancer at a relatively old age.
The most common genetic syndromes associated with colorectal cancer:
- familial adenomatous polyposis and its variants,
- Lynch syndrome,
- Peutz-Jeghers syndrome,
- juvenile polyposis.
Pathology[edit | edit source]
Molecular pathology[edit | edit source]
From a molecular point of view, colorectal cancer is a group of several different diseases in which there is some overlap. This is because a malignant reversal can occur due to several mutation sequences. Five types of colorectal cancer can be distinguished based on molecular characteristics. Individual types differ according to CpG islet hypermethylation (CIMP), microsatellite instability (MSI, MSS = stable), chromosomal aberrations and characteristic mutations:[11]
- Type 1 CIMP-H, MSI-H, BRAF mutations, chromosomes stable. It accounts for about 12% of colorectal cancers, also referred to as sporadic MSI-H colorectal cancer. It arises on the basis of serous lesions.
- Type 2 CIMP-H, MSI-L or MSS, BRAF mutations, chromosomes stable. It represents about 8% of colorectal cancers, it arises on the basis of serial lesions.
- Type 3 CIMP-L, MSI-L or MSS, KRAS mutations, chromosomal instability. It represents about 20% of colorectal cancers. It arises on the basis of serous lesions and classic adenomas.
- Type 4 CIMP-negative, MSS, chromosomes unstable. It arises on the basis of a congenital or acquired mutation of APC or MUTYH, it arises from classical adenomas. It represents about 57% of colorectal cancers.
- Type 5 CIMP-negative, MSI-H, chromosomes are stable. It is caused by Lynch syndrome, sometimes referred to as familial MSI-H cancer. It represents about 3% of colorectal cancers.
In sporadic colorectal cancers, three major areas of change in cell biology can be distinguished, and there are three ways to divide them:[12]
- chromosomal instability pathway,
- microsatellite instability pathway,
- hypermethylation of CpG islands pathway.
Chromosomal instability pathway[edit | edit source]
Chromosomal instability is the most common cause of gene instability in colorectal cancers, which can be demonstrated in 65–70% of all cases. It involves a broad change in chromosomal changes, which can result in both amplification of some genes and loss of heterozygosity of other genes. The following changes most commonly occur in the following locations:
- Extensive gain: 7, 8q, 13q, 20, X.
- Extensive loss: 1, 4, 5, 8p, 14q, 15q, 17p, 18, 20p, 22q.
- Focal gain / loss in the field of genes: VEGF, MYC, MET, LYN, PTEN.
- Numerous allele losses: 1, 5, 8, 17, 18.
- Whole chromosome loss: 18.
The most significant molecular changes at the single gene level are the APC, MCC and K-ras mutations, at the level of the larger regions the cleats at 5q, 8p, 17p and 18q.[12]
- APC
APC is a tumor suppressor gene whose innate mutation is responsible for most cases of familial adenomatous polyposis. The product of the gene is a multifunctional 310 kDa protein involved in epithelial homeostasis mainly by regulating the degeneration of cytoplasmic β-catenin and thus a key part of the wnt signaling cascade. APC is also involved in cell cycle control and microtubule stabilization.
- MCC
MCC is a gene whose product is involved in cell cycle arrest in DNA damage and apparently interferes with the Wnt signaling cascade. In colorectal cancers, its expression is often suppressed by promoter hypermethylation, direct mutations are not common.
- TP53
TP53 is a tumor suppressor gene whose product, p53, is a transcription factor that plays a central role in regulating cell cycle progression depending on the detection of DNA damage. Mutation of the TP53 gene is a relatively characteristic event in the late tumorigenesis of colorectal cancer.
- K-ras
K-ras is an oncogene whose product is a membrane protein with GTPase activity involved in signaling in a number of signaling cascades. The mutation leads to a permanent activation, which in turn leads to an increase in the transcriptional activity of a number of genes, in particular BCL-2, H2AFZ, RAP1B, TBX19, E2F4 and MMP1. Numerous pathways regulating cell growth, proliferation, apoptosis, cytoskeletal organization, and cell motility are thus affected by increased K-ras activity. The K-ras mutation is thought to play a crucial role in the transition from adenoma to cancer.
- Loss of 5q
Loss of 5q occurs in 20-50% of sporadic colorectal cancers. The APC and MCC genes are mainly found in this area.
- Loss at 8p
Loss at 8p occurs in approximately 50% of colorectal cancers. Proof of loss is more common in the advanced stage, this aberration is less common in the earlier stages. Loss in the 8p region increases the metastatic potential of colorectal cancer. Candidate genes are found mainly in the 8p21 and 8p22 regions.
- Loss at 17p
Losses in the 17p region occur in 75% of colorectal cancers, but do not occur in adenomas. This region contains the p53 gene.
- Loss at 18q
The long arm of chromosome 18 contains a number of tumor suppressor genes as well as genes involved in the control of cell adhesion and migration.
Microsatellite instability pathway[edit | edit source]
Microsatellites are short repetitive sequences that occur throughout the genome. Their instability is a "macroscopic" manifestation of a "mismatch repair" system failure. At the transcriptional level, microsatellite instability is manifested by a frameshift. Carcinogenesis associated with microsatellite instability is associated with mutations in a number of mismatch repair system genes: MSH2, MLH1, MSH6, PMS2, MSH3, PMS1 and Exol; germline mutations in some of these genes cause Lynch syndrome.
Since 1997, five loci have been recommended for microsatellite instability analysis: the mononucleotide repetitive sequences BAT25 and BAT26 and the dinucleotide repetitive sequences D5S346, D2S123 and D17S250. According to the evidence of instability, there are three phenotypes of microsatellite instability:
- MSI-high (MSI-H) - instability in at least two of these loci,
- MSI-low (MSI-L) - instability in just one locus,
- MSS - all five tested loci are stable.
Alternatively, BAT25, BAT26, NR21, NR24 and NR27 loci are used, the evaluation of which appears to be more consistent with mismatch repair gene disorders.
Another possibility for molecular evaluation is the analysis of the instability of selected tetranucleotide repetitive sequences. The phenotype with increased instability is called EMAST, which corresponds to the suppression of MSH3 gene expression.
MSI-H tumors are often diploid, loss of heterozygosity occurs less frequently, and p53 and K-ras mutations are less common. The V600E mutation of the BRAF gene is common in sporadic MSI-H tumors. Inactivating mutations in the TGFβRII receptor gene (TGFBR2 gene) are very common in tumors with MSI; this inactivation leads to the elimination of antiproliferative signaling by the cytokine TGF-β.[12] Mutations inactivating the antiproliferative cascade beginning with the TGFβRII receptor are considered to be a significant step from adenoma to high-grade dysplasia resp. to cancer.[13]
Hypermethylation of CpG islands pathway[edit | edit source]
A characteristic feature of this pathway are epigenetic changes, specifically cytosine hypermethylation in CpG sequences. Hypermethylation in the promoter regions of tumor suppressor genes is pathogenetic, which induces a decrease in their expression. Hypermethylation usually occurs in the promoter sequences of the APC, MCC, MLH1 and MGMT genes. It should be noted that it is not yet clear what is the cause of aberrant hypermethylation.[12][13]
Evaluation of CpG sequence methylation in five marker genes is used to analyze hypermethylation: CACNA1G, IFG2, NEUROG1, RUNX3, SOCS1. There are two levels of CpG islet methylation:
- CIMP-high (CIMP-H) - evidence of methylation in at least three markers
- CIMP-low (CIMP-L) - evidence of methylation in less than three markers.
The CIMP-H phenotype is often associated with the BRAF mutation.[12]
Influence of growth factors[edit | edit source]
Growth factors from the environment also contribute to a certain extent to the origin and growth of first adenoma and later also carcinoma.
- Prostaglandins
Prostaglandin E2 (PGE2) is very strongly associated with colorectal cancer. Cyclooxygenase COX-2 is involved in its synthesis, and increased COX-2 activity has been shown in about two-thirds of tumors. Degradation of PGE2 is performed by 15-prostaglandin dehydrogenase, about 80% of tumors have a defect in this enzyme.[13]
- EGF
EGF (Epidermal Growth Factor) is a cytokine that is involved in growth signaling. EGFR blockade is one of the possible therapeutic modalities for advanced colorectal cancer, the blockade is ineffective in tumors with activating mutations in the EGFR pathway, especially mutations KRAS, BRAF and the p110 phosphatidylinositol kinase (PI3K) subunit.[13]
- VEGF
VEGF (Vascular Endothelial Growth Factor) is a cytokine that stimulates vascularization during growth and neovascularization of healing tissues. It is also used in vascularization and tumor growth, its blockade is one of the possible therapeutic modalities of advanced colorectal cancer.[13]
Macroscopic appearance[edit | edit source]
More than half of cancers occur in rectosigmoid, and right-sided tumors are more common in elderly patients and in patients with diverticulosis. Multicenter incidence is uncommon, with reported incidence between three and six percent.
Macroscopically, colorectal cancer can present as a polyposis (exophytic) or as a hollow (exulcerated) lesion. Polypous carcinoma protrudes above the level of the mucosa, its margins are relatively steep and it is usually well defined macroscopically. The excavated carcinoma has elevated margins, and is indented and exulcerated in the middle. A variant of excavated cancer is flat (infiltrating) cancer. In the right colon, the tumor may surround the wall (carcinoma anulare) and lead to stenosis.
- Macroscopic appearance of colorectal cancer
Metastatic colorectal cancer spreads mainly through the lymphatic route to regional lymph nodes. Due to the arrangement of lymphatic vessels in the mesentery, lymphogenic metastasis can be detected quite often even in the nodes seemingly draining areas of the intestine far from the tumor. Hematogenous metastases usually occur in advanced disease. The liver is primarily affected, and other sites are uncommon. If the tumor grows into the peritoneum, porogenic metastases after the peritoneum are also possible.
Histopathology[edit | edit source]
Histologically, colorectal cancer is in more than 90% of cases adenocarcinoma, other histological types are:
- neuroendocrine tumor,
- squamous cell carcinoma,
- adenosquamous carcinoma,
- spindle cell carcinoma,
- undifferentiated carcinoma.
The basis for adenocarcinoma grading is the evaluation of glandular formation:
- Well-differentiated adenocarcinoma is characterized by glandular formation accounting for more than 95% of the tumor. This grade represents about 10% of all colorectal adenocarcinomas.
- Moderately differentiated adenocarcinoma is characterized by glandular formation accounting for 50-95% of the tumor. This grade is the most common, accounting for about 70% of all colorectal adenocarcinomas.
- Poorly differentiated adenocarcinoma is characterized by the fact that the glandular formation makes up less than 50% of the tumor. This grade represents about 20% of all colorectal adenocarcinomas.
Three-level grading is burdened by a relatively large share of subjective evaluation. Therefore, some authors recommend grading with only two levels, which has less variability of evaluation between different pathologists and which should also have a better informative value of the forecast pages:
- Low grade adenocarcinoma means that glandular formations make up at least 50% of the tumor.
- High grade adenocarcinoma means that the glandular formation makes up less than 50% of the tumor.
This grading can only be used for conventional adenocarcinoma; in the case of its histological changes, even a high-grade appearance can be associated with prognostically more favorable behavior.[14]
Identifying signs of invasion is key to assessing biological behavior. If muscularis mucosae is trapped in the mount, which may not be the rule for endoscopically collected specimens, it should be assessed whether it is damaged by the tumor. Invasive carcinoma grows through the muscular mucosae into the submucosa, where it may be closely related to the submucosal vessels.
Another important sign of invasive behavior is desmoplasia resp. desmoplastic reaction, a type of ligament proliferation induced by invasive tumor growth. The desmoplastic reaction is characterized by the proliferation of spindle cells that surround the tumor gland.
A unique and relatively common feature of colorectal cancer is necrotic detritus in the lumen of the tumor glands, sometimes referred to as dirty necrosis. These can also occur in metastases, so they are an important guide in determining the origin of tumors of unknown origin.
In the case of colorectal cancer, only submucosal invasive cancer should be designated as invasive carcinoma, i.e. the pT1 stage. Furthermore, tumors growing into the muscularis mucosae without growth have little potential to establish distant metastases or lymph node metastases. The biological reason for such behavior of the tumor growing into the muscularis mucosae is not exactly known, it is assumed that a relatively poor network of lymphatic vessels plays a role. According to the AJCC Cancer Staging Manual (7th edition), the tumor that grows highest to the muscularis mucosae should be referred to as carcinoma in situ (pTis), and some authors even recommend high-grade dysplasia so that the terminology does not give the impression that further surgery is needed.[14]
- Microscopic appearance of colorectal adenocarcinoma
Colorectal adenocarcinoma can have several histological variants:
- mucinous adenocarcinoma,
- adenocarcinoma with ring-shaped cells,
- medullary adenocarcinoma,
- micropapillary adenocarcinoma,
- serrated adenocarcinoma,
- cribriform comedo adenocarcinoma,
- adenosquamous adenocarcinoma,
- spindle cell adenocarcinoma,
- undifferentiated adenocarcinoma.
- Mucinous adenocarcinoma
A defining feature of mucinous adenocarcinoma is that at least 50% of the tumor volume is extracellular mucin. Carcinomas that contain less mucin but still more than 10% by volume are referred to as adenocarcinoma with mucinous differentiation or adenocarcinoma with mucinous properties.
Histologically, mucinous adenocarcinomas usually form relatively large glandular formations with pools of extracellular mucin. Different numbers of individual tumor cells may be present, and seal ring-shaped cells may be captured.
The biological behavior of mucinous adenocarcinoma is uncertain. Lynch syndrome has a microsatellite phenotype, MSI-H, and its behavior can be assessed as low-grade. On the other hand, when mucinous adenocarcinoma is associated with the microsatellite phenotype of MSS, its biological behavior is clearly more aggressive.[14]
- Adenocarcinoma with ring-shaped cells
A defining feature of seal ring-shaped cell adenocarcinoma is that at least 50% of the tumor cells have the features of seal ring-shaped cells. A characteristic feature of the seal ring-shaped cells is the bulky central vacuole filled with mucus and pushing the nucleus to the edge of the cell. Infiltrative growth or uptake of free cells in extracellular mucin pools is common. Seal-ring cell adenocarcinoma usually behaves like a high-grade tumor, but low-grade behavior can be expected if the MSI-H phenotype is demonstrated.[14]
- Medullary adenocarcinoma
Medullar adenocarcinoma is characterized by strips of epithelioid cells with bulky vesicular nuclei, distinct nucleoli and abundant cytoplasm. Tumor infiltration by abundant lymphocytes is relatively common. Cell differentiation is usually poor, cells can be undifferentiated. The microsatellite phenotype MSI-H is relatively common. Despite the usually poor differentiation, the prognosis is relatively good.[14]
Clinical management[edit | edit source]
Clinical picture[edit | edit source]
Colorectal cancer, especially right-sided cancer, is often clinically silent in the early stages and can only be detected at screening. Typical manifestations are bleeding into the colon and changes in bowel movements (eg alternating diarrhea and constipation). Chronic blood loss can lead to anemia. Another relatively common manifestation is non-specific and rather vague abdominal pain. On the left side and less often in the colonic ascendant and in the cecum, colonic obstruction may be one of the first manifestations of the tumor.
Screening[edit | edit source]
Early detection of colorectal cancer is a factor that significantly improves the patient's prognosis. The aim of screening is not only to detect an existing malignancy, but also premalignant changes. The optimal timing of individual screening methods is a matter of ongoing research.[5]
Occult bleeding[edit | edit source]
Fecal Occult Blood (FOB) is a condition in which there is an admixture of blood in the stool that exceeds the physiological blood loss to the stool. The test is relatively cheap, not burdensome, but it is less sensitive. Nevertheless, its regular implementation every two years has the potential to reduce colorectal cancer-related mortality by up to 16%.[5]
Sigmoidoscopy[edit | edit source]
Examination of the rectosigmoid with a flexible 60 cm sigmoidoscope allows the detection of about 60% of colorectal cancers. The advantage over colonoscopy is easier preparation of the patient, an enema is sufficient for the procedure, and also a lower risk of complications.[5]
Colonoscopy[edit | edit source]
Colonoscopy is the gold diagnostic standard because, in addition to overlooking the mucosa of virtually the entire colon, it also allows for sampling of suspected lesions and the excretion of premalignant lesions.
Virtual colonoscopy[edit | edit source]
Virtual colonoscopy (CT colonography) may be suitable for assessing the exact location of the tumor, especially in cases where colonoscopy cannot be performed. The disadvantage is the radiation exposure of the patient.
Capsule colonoscopy[edit | edit source]
Capsule colonoscopy is a screening method that involves swallowing a capsule capable of taking pictures of the digestive tract. The main advantage compared to conventional colonoscopy is higher patient comfort (and thus higher compliance) and lower risk of complications, the disadvantage is the inability to take a sample for histological examination and the time required to evaluate the record. If a suspected lesion is detected, a classic colonoscopy should be performed.[15]
Molecular biological tests[edit | edit source]
In the research phase, screening tests are based on the analysis of DNA in blood or stool.[5]
Diagnostics[edit | edit source]
The diagnosis is determined on the basis of sigmoidoscopic resp. colonoscopy with histological examination. The newly diagnosed tumor should be followed by additional tests (if not already performed):[5]
- physical exam,
- complete colonoscopy to rule out metachronous cancer elsewhere in the colon,
- CT examination of the lungs, abdomen and pelvis to detect possible metastatic disease.
In rectal cancer, MR examination is more effective, especially with regard to assessing the spread of the tumor into the mesorectum and thus to assess the appropriate extent of resection. In the case of early tumors, endoscopic ultrasound can be used to assess invasion. Both of these methods have their advantages and limitations. Ultrasound examination is advantageous for the examination of the liver, and the use of ultrasound contrast agents seems promising. Peritoneal carcinomatosis remains a diagnostic problem.[5]
ICD[edit | edit source]
- The primary localization is determined by several ICD-10 codes
- C18, C19, C20, C21
- C18: Malignant neoplasm of colon.
- C18.0: Cecum and ileocecal valve.
- C18.1: Appendix.
- C18.2: Colon ascendens.
- C18.3: Hepatic flexure
- C18.4: Transverse column.
- C18.5: Linear flexure.
- C18.6: Descendant Colon.
- C18.7: Colon sigmoid.
- C18.8: The lesion extends beyond the intestine.
- C18.9: Not specified.
- C19: Malignant neoplasm of rectosigmoid junction.
- C20: Malignant neoplasm of rectum.
- C21: Malignant neoplasm of anus and anal canal.
- C21.0: Anus, unspecified.
- C21.1: Anal canal.
- C21.2: Cloacogenic zone.
- C21.8: The lesion extends beyond the rectum, anus and anal canal.
Staging[edit | edit source]
| TNM classification of colorectal cancer
Primary tumor size | |
|---|---|
| TX | the primary tumor could not be evaluated |
| T0 | primary tumor not found |
| Tis | intraepithelial growth or invasion of the lamina propria |
| T1 | invasion of submucosa |
| T2 | invasion of the muscular propria |
| T3 | invasion through the muscularis mucosae into the surrounding tissues |
| T4a | penetration of the visceral peritoneum surface |
| T4b | penetration into surrounding organs |
| Lymph node involvement | |
| NX | lymph nodes could not be evaluated |
| N0 | lymph nodes without metastases |
| N1 | metastases in one to three regional nodes |
| N1a | metastasis in one regional node |
| N1b | metastases in two to three regional nodes |
| N2 | metastases in four or more regional nodes |
| N2a | metastases in four to six regional nodes |
| N2b | metastases in seven or more regional nodes |
| Distant metastases | |
| MX | distant metastases could not be evaluated |
| M0 | without evidence of distant metastases |
| M1 | distant metastases |
| M1a | distant mesastases in one anatomical locality |
| M1b | distant metastases in multiple anatomical localities |
At least 12 lymph nodes should be examined for adequate lymph node involvement.[5] Technical conditions sometimes do not allow this, and therefore a sign of worse pathology work is considered only if the average number of examined nodes in a larger number of resections drops significantly below 12.
- Clinical stages
- stage 0: TisN0M0
- stage I: T1N0M0 or T2N0M0
- stage IIA: T3N0M0
- stage IIB: T4N0M0
- stage IIIA: T1N1M0 or T2N1M0
- stage IIIB: T1N2M0, T2N2M0 or T3N1M0
- stage IIIC: T3N2M0, T4N1M0, T4N2M0
- stage IVA: any T and N, distant metastases in one anatomical locality
- stage IVB: any T and N, distant metastases in more than one anatomical locality
- Dukes classification
- stage A: tumor bounded by the intestinal wall
- stage B: the tumor invades or penetrates serosa
- stage C: lymph node involvement
- stage C1: positive pericolic lymph nodes
- stage C2: positive perivascular nodes
- stage D: distant metastases
Typing a grading[edit | edit source]
Typing and grading are governed by the WHO classification of colorectal tumors (incl. ICD-O codes, abbreviated):[16]
- Carcinomas:
- adenocarcinoma 8140/3
- mucinous adenocarcinoma 8480/3
- seal ring cell carcinoma 8490/3
- small cell carcinoma 8041/3
- squamous cell carcinoma 8070/3
- adenosquamous carcinoma 8560/3
- medullary carcinoma 8510/3
- undifferentiated carcinoma 8020/3
- Carcinoid 8240/3
- EC cells, serotonin producing neoplasia 8241/3
- Mixed carcinoid-adenocarcinoma 8244/3
- Non-epithelial tumors
- gastrointestinal stromal tumor 8936/1
- leiomyosarcoma 8890/3
- angiosarcoma 9120/3
- Kaposi's sarcoma 9140/3
- malignant melanoma 8720/3
- lymphoma
Therapy[edit | edit source]
Surgical therapy[edit | edit source]
Surgical therapy is a basic therapeutic modality. If it is chosen, it is appropriate to perform a sufficiently radical performance, incl. lymphadenectomy. The resection margin should be at least 5 cm from the tumor if possible; some authors consider a distal edge of the rectal resection of 2 cm to be sufficient. The extent of resected adipose tissue should be such that at least 12 lymph nodes can be examined. In the case of T4, only a sufficiently large resection "en bloc" can be considered as a resection with intact resection margins. Mechanical contusion of the tumor during resection can spread it, and therefore the technique of surgery is adapted to prevent such spread - the no-touch concept. If the tumor is traumatized during the procedure, adjuvant chemotherapy should be used.[4][5]
For invasive rectal cancer, the method of choice is total mesorectal excision (TME) with adequate circular and distal resection margins and excision of the lower mesenteric lymph nodes. The sphincter-saving procedures are intended for patients in the lower stage of the disease; in the very early stages (T1Sm1), local exicciation can also be considered.[5]
Laparoscopic colectomy is the method of choice for colon tumors. From the oncological point of view, the results of the laparoscopic procedure can be considered identical to the laparotomy procedures, the laparoscopic procedure is more advantageous for the patient.[5]
Resection of liver and lung metastases, if established, significantly increases patient survival. In addition to surgical resection, eg local embolization or radiofrequency ablation is used; these methods increase the number of patients who can be treated for metastases. Neoadjuvant chemotherapy may also target metastases.[5]
Palliative procedures, especially bypass surgery and derivation stoma, are possible for unresectable tumors or tumors of patients not operable for comorbidities. These procedures can improve the patient's quality of life and, in conjunction with appropriate pharmacotherapy, survival.[4]
Radiotherapy[edit | edit source]
Neoadjuvant radiotherapy allows surgery to be performed in patients in a more advanced stage, but does not in itself affect overall survival.[4]
Pharmacotherapy[edit | edit source]
Neoadjuvant chemotherapy is usually given with 5-fluorouracil, sometimes combined with radiotherapy.[17]
Adjuvant chemotherapy improves survival in II. stage by a few percent, in III. stage by 15-20%. For this reason, in III. stage adjuvant chemotherapy is fully indicated. In addition to 5-fluorouracil, oxaliplatin is also used. Biological therapy is not indicated in adjuvant therapy.[17]
Therapy IV. stage is a complex matter, the procedure is chosen according to the patient's condition and the extent of metastatic disability. In addition to cytostatics (5-fluorouracil, oxaliplatin, irinotecan, fluoropyrimidine), biological therapy can also be used if its results can be expected from the results of molecular examination. Currently available:[17]
- EGFR receptor inhibitors cetuximab and panitumumab,
- bevacizumab anti-VEFG antibody,
- aflibercept - circulating VEGF binding protein,
- protein kinase inhibitor regorafenib signaling cascade series.
Prognosis[edit | edit source]
The prognosis of adenocarcinoma is similar in cancer of the rectum and colon, depending mainly on the clinical stage. Five-year survival is as follows:[5]
- stage I: 97.1%
- stage IIA: 87.5%
- stage IIB: 71.5%
- stage IIIA: 87.7%
- Stage IIIB: 75.0% (if N1), 68.7% (if N2)
- Stage IIIC: 47.3% (T3, N2), 50.5% (T4, N1), 27.1% (T4N2)
In the fourth stage, the prognosis depends on which organs are metastatically affected, what is the extent of the metastatic disability and what is the overall condition of the patient. Overall, however, the prognosis is not very favorable and at this stage colorectal cancer is usually considered incurable in terms of curative therapeutic intent in the choice of therapeutic modalities.[13]
Links[edit | edit source]
Source[edit | edit source]
Reference[edit | edit source]
- ↑ Jump up to: a b STROUHAL, E. a A. NĚMEČKOVÁ. Trpěli i dávní lidé nádoy? : Historie a paleopatologie nádorů, zvláště zhoubných. 1. vydání. Praha : Karolinum, 2008. ISBN 9788024614816.
- ↑ Jump up to: a b c d e f g h i MULCAHY, H.E., J. HYLAND a D.P. O'DONOGHUE. From dinosaurs to DNA: a history of colorectal cancer. Int J Colorectal Dis. 2003, vol. 18, no. 3, s. 210-5, ISSN 0179-1958.
- ↑ CRAWFORD, K., et al. European Sexualities, 1400-1800. 1. vydání. Cambridge University Press, 2007. 246 s. New Approaches to European History; sv. 38. Kapitola 5 John Burrows and the Vegetable Wars. s. 85–102. ISBN 9780521839587.
- ↑ Jump up to: a b c d KALA, Z., P. KYSELA a L. OSTŘÍŽKOVÁ, et al. Chirurgická a miniinvazivní léčba kolorektálního karcinomu. Onkologie [online]. 2011, vol. 5, no. 5, s. 270-272, dostupné také z <http://www.onkologiecs.cz/pdfs/xon/2011/05/07.pdf>. ISSN 1803-5345.
- ↑ Jump up to: a b c d e f g h i j k l m n CUNNINGHAM, D., W. ATKIN a H.J. LENZ, et al. Colorectal cancer. Lancet. 2010, vol. 375, no. 9719, s. 1030-47, ISSN 1474-547X.
- ↑ LAZARUS-BARLOW, W.S. a J.H. LEEMING. The natural duration of cancer. Br Med J [online]. 1924, vol. 2, no. 3320, s. 266-7, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2304825/?tool=pubmed>. ISSN 0007-1447.
- ↑ WYARD, S.. The natural duration of cancer. Br Med J [online]. 1925, vol. 1, no. 3344, s. 206-7, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2196841/?tool=pubmed>. ISSN 0007-1447.
- ↑ Jump up to: a b G. VEPŘEKOVÁ a O. MÁLEK, et al. Epidemiologie, etiologie, screening a diagnostika kolorektálního karcinomu, včetně diagnosticko-terapeutických zákroků na tlustém střevě. Onkologie [online]. 2011, vol. 5, no. 5, s. 261-265, dostupné také z <http://www.onkologiecs.cz/pdfs/xon/2011/05/05.pdf>. ISSN 1803-5345.
- ↑ http://gco.iarc.fr/today/fact-sheets-populations
- ↑ http://www.svod.cz/
- ↑ JASS, J.R.. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology.. 2007, vol. 50, no. 1, s. 113-30, ISSN 0309-0167.
- ↑ Jump up to: a b c d e In sporadic colorectal cancers, three major areas of change in cell biology can be distinguished, and there are three ways to speak:
- ↑ Jump up to: a b c d e f MARKOWITZ, S.D. a M.M. BERTAGNOLLI. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med [online]. 2009, vol. 361, no. 25, s. 2449-60, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843693/?tool=pubmed>. ISSN 1533-4406.
- ↑ Jump up to: a b c d e FLEMING, M., S. RAVULA a S.F. TATISHCHEV, et al. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol [online]. 2012, vol. 3, no. 3, s. 153-73, dostupné také z <http://jgo.amegroups.com/article/view/410/html>. ISSN 2219-679X.
- ↑ M. VOŠKA a G. VEPŘEKOVÁ, et al. Kolonická kapsle v kontextu screeningu kolorektálního karcinomu. Onkologie [online]. 2012, vol. 6, no. 3, s. 165-168, dostupné také z <http://www.onkologiecs.cz/pdfs/xon/2012/03/08.pdf>. ISSN 1803-5345.
- ↑ HAMILTON, Stanley R. a Lauri A. AALTONEN. WHO Classification of Tumours : Pathology and Genetics of Tumours of the Digestive Syste, [online] . 1. vydání. Lyon : IARC Press, 2000. Dostupné také z <http://publications.iarc.fr>. ISBN 92-832-2410-8.
- ↑ Jump up to: a b c a J. HALÁMKOVÁ. Systémová protinádorová léčba kolorektálního karcinomu. Onkologie [online]. 2013, vol. 7, no. 4, s. 188-189, dostupné také z <http://www.onkologiecs.cz/pdfs/xon/2013/04/07.pdf>. ISSN 1803-5345.
Virtual preparations[edit | edit source]
| source: Juan Rosai's Collection | ||
Related articles[edit | edit source]
- Colorectal carcinoma
- Treatment of liver metastases in colorectal cancer
- Colon polyps
- Vienna Classification of Gastrointestinal Neoplasias (2002)
Literature[edit | edit source]
- ROSAI, Juan. Ackerman's Surgical Pathology. 8th edition. St. Louis, MO: Mosby, 1996. vol. 1. ISBN 0-8016-7004-7.
- HAMILTON, Stanley R. and Lauri A. AALTONEN. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Digestive System, [online]. 1st edition. Lyon: IARC Press, 2000. Also available from <http://publications.iarc.fr>. ISBN 92-832-2410-8.
- POVÝŠIL, Ctibor and Ivo ŠTEINER, et al. Special pathology. 2nd edition. Prague: Galén: Karolinum, 2007. ISBN 978-80-7262-494-2.
External links[edit | edit source]
- Dušek, L., Zavoral, M., Májek, O., Suchánek, Š., Mužík, J., Pavlík, T., Šnajdrová, L., Gregor, J. Kolorektum.cz – Program kolorektálního screeningu v České republice [online]. Masarykova univerzita, Brno, 2014. Dostupné z WWW: [7]. ISSN 1804-0888.
- VYZULA,., et al. Modrá kniha České onkologické společnosti [online] . 19. vydání. 2014. Dostupné také z <https://www.linkos.cz/files/modra-kniha/12.pdf>. Doporučení České onkologické společnosti ČLS JEP. ISBN 978-80-86793-35-1.
- MARKOWIT.D. a M.M. BERTAGNOLLI. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med [online]. 2009, vol. 361, no. 25, s. 2449-60, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843693/?tool=pubmed>. ISSN 1533-4406.