Conductometry
Conductometry is one of the oldest electroanalytical methods, which deals with measuring of the conductivity of electrolyte solutions in water or in another solvent with a high relative permitivity, where dissociation of the electrolyte into ions occurs. Conductometry is base on the ability of the electrolyte solution to conduct an electric current, and the conductometric cell consists of two metal, most often platinum, electrodes immersed in the analyzed solution. At the same time, in low-frequency conductometry, alternating current is used for measurement, which prevents the platinum electrodes from being polarized.
The conductivity value of the electrolyte depends on
- migration of ions present in the solution to the electrodes, i.e. on mobility
- the number of ions an their charge
and is characterized by specific conductivity X (unit: mS.m−1).
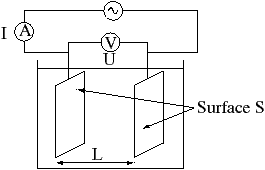
X = 1/R. l/S
Since the conductivity G = 1/R (siemens conductivity unit S) and the resistance constant of the conductivity container KR = l/S (l = vzdálenost, S = plocha elektrod), i.e.
X = G. KR
The conductivity container is realized by two non-polarizable platinum electrodes - plates with an area of 1 cm2 built into a glass at a distance of 1 cm. In this case, the KR value is unitary and the measured value therefore directly corresponds to the specific conductivity.
The conductivity of solutions can be measured by the method[edit | edit source]
1. compensatory, the essence of which is the use of a Wheatstone bridge. This method of measurement using a zero indicator allows accurate resistance measurement, but is not suitable for titrations. Instruments that measure in this compensatory way are referred to as conductoscopes.
2. deflection, which makes it possible to measure the resistance between the electrodes of the conductivity container in the current state. Instruments − conductometers enable fast and direct reading of conductivity values. The measurable conductivity range is from 0,1 μS to 1 S, the entire range is divided into selectable sub-ranges; accuracy to about 1%.
Direct conductometry[edit | edit source]
Direct conductometry makes it possible to determine the concentration of the electrolyte based on the conductivity measurement of the solution. However, its use in analytical chemistry is limited because the conductivity of most ions, with the exception of H3O+ and OH−, differs little, and conductivity is a non-specific, additive property. Therefore, this method is not suitable for determination in more complex solutions. The advantage, however, is the high sensitivity of conductometry, which makes it possible to determine even trace amounts of electrolytes in water. That is why primary conductometry is used especially when checking the purity of water. Specific conductivity is one of the main indicators of the quality of e.g. distilled water.
According to the pharmacopoeia, the calibration of the conductometric system is performed with a potassium chloride solution with a concentration of 0,02 mol.l−1, the specific conductivity of which is 250,1 mS.m−1.
On the other hand, the non-specific character of conductivity is advantageously used in conductometric detectors of some instrumental methods, such as high-performance liquid chromatography or isatochophoresis.
Conductometric titration[edit | edit source]
In conductometric titrations, the change in conductivity of the solution during the titration is used to identify the equivalence point. The conductometric titration curve is the dependence of specific conductivity or total conductivity on the added volume of measuring solution. The graphical representation of this dependence is two straight branches, one before and the other after reaching the equivalence point, whose intersection corresponds to the consumption of the measuring solution at the equivalence point.
A conductometric titration is performed similarly to a potentiometric titration by adding the measuring solution in portions and after each addition and mixing, the reading of consumption and conductivity is recorded. In doing so, it is enough to measure 3-4 points before and after the equivalence point, after their graphical representation, fit straight lines and subtract the equivalence point. In order not to introduce a correction for the dilution caused by the added measuring solution, it is necessary to titrate with a solution at least ten times more concentrated thatn the concentration of the determined substance in the solution.
Conductometric titration is of greates importance in the determination of acids and bases. In the direct titration of acids, the titration curve has a V shape, which is due to the fact that the molar conductivity of the reacting ions H3O+ a OH− is much greater than the conductivity of other ions, and the resulting water has a negligible conductivity. For the determination of weak acids and bases and very dilute solutions, conductometric titration (identification of the equivalence point) is preferable to potentiometric titration. Another method is the determination of ammonium salts of acids by titration with hydroxide. The advantage of this method is that even acids that are poorly soluble in water dissolve in the excess of ammonia, and in this way it is possible to determine e.g. imides, esters and anhydrides of acids in addition to acids.
Conductometric indications of the equivalence point can also be used in many precipitation titrations. Hydroxide or e.g.: 2,3-butandione dioxime is used as a measuring solution for the determination of cations; when determining anions, for example, sulfate is titrated with barium acetate, halides with silver acetate, etc. Similarly, conductometric indications can also be used in the chelatometric determination of diluted solutions of cations with chelate 3, while the hydrogen ions formed are bound with acetate. Conductometric indication is unsuitable for redox titrations.
Links[edit | edit source]
References[edit | edit source]
- KARLÍČEK, Rolf, et al. Analytická chemie pro farmaceuty. 3rd edition. Praha : Karolinum, 2007. ISBN 9788024614533.