Congenital malformations of the respiratory system
Congenital malformations of the airways often endanger the life of a newborn and require urgent surgical treatment, or they may remain unrecognized for a long time and cause recurrent and chronic bronchopulmonary diseases.
Choanal stenosis and atresia are only exceptionally separate congenital malformations, they usually occur together with other congenital malformations (gothic palate, septal deviation, congenital heart defects, spinal defects, or intellectual disorders). It can be bone or membranous. It is the most common congenital anomaly in the nose area, less severe unilateral atresia is manifested by reduced breathing, drinking difficulty, and purulent and grumous secretion from the nose.
Clinical picture of bilateral atresia: immediately after birth, the baby suffocates (the newborn breathes only through the nose), has cyanosis. In milder forms, the difficulties are exacerbated by drinking, there is a risk of food aspiration (it can kill the newborn). Diagnosis: inability to insert the probe through the nasal cavity into the pharynx (max. 5 cm), X-ray with contrast in lateral projection.
Surgical treatment. First aid: keep mouth open (using an oropharyngeal tube or endotracheal intubation).
Congenital malformations of the larynx[edit | edit source]
Atresia (or stenosis) of larynx[edit | edit source]
A rare anomaly causing upper airway obstruction in a fetus. Distal to the atresia/stenosis, the airways are dilated, the lungs are enlarged – the tap is echogenic, the diaphragm is flat to inverted, fetal ascites or hydrops are developed. Can be recognized by ultrasonography.
Subglottic stenosis can be congenital, but more often it is iatrogenic (e.g. after long intubation), rarely it can be caused by a tumor or hemangioma. Endoscopic examination is required for a definitive diagnosis.
Rete laryngis, laryngeal diaphragm[edit | edit source]
This rare anomaly is due to incomplete laryngeal recanalization during the 10th week. At the level of the vocal cords, a membraneous network (rete) is formed, which partially closes the airways.
The laryngeal diaphragm is located at or below the vocal cords, most often in the anterior commissure. It is about 5% the cause of congenital stridor. The difficulty depends on the strength and size of the membrane. Unlike malacia, dysphonia and barking cough often occur.
Laryngomalacia (stridor laryngis congenitus)[edit | edit source]
Congenital laryngeal stridor (stridor laryngis congenitus) or laryngomalacia is caused by the retarded development of laryngeal cartilage (mainly epiglottis), which leads to their abnormal softness. Epiglottis, together with aryepiglottic folds, is sucked into the laryngeal entrance during inspiration, which causes obstruction of the upper respiratory tract.
It manifests clinically immediately after birth or in the first weeks by inspirational stridor, it improves in the abdominal position. It can be constant or intermittent. As a rule, stridor worsens with crying, restlessness, or infection. Congenital stridor is evidenced by the fact that it eases or disappears when the child's mandible is pushed forward.
Specific therapy is not necessary, for children there is preferably an elevated position (especially when feeding). It usually adjusts spontaneously within a year.
Congenital malformations of the trachea[edit | edit source]
Congenital stenosis of the trachea[edit | edit source]
Congenital stenosis of the trachea can be diffuse, funnel-shaped (in are of bifurcation), or segmental. Clinical picture: stridor, dyspnoea, recurrent respiratory infections, can cause obstructive emphysema. Diagnosis: X-ray, tracheoscopy.
Therapy: sometimes tracheostomy is necessary. Difficulties may improve with age. Reconstruction procedures, an intraluminal stent.
Tracheomalacia[edit | edit source]
Tracheomalacia is the increased compliancy of cartilage or its complete absence and replacement by a membrane, which causes the wall to collapse during exhalation. It can only manifest itself when hard exhaling or coughing. Diagnosis is endoscopic, treatment is usually not necessary.
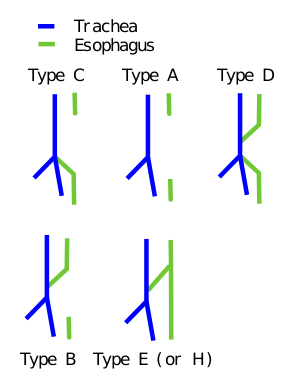
Tracheoesophageal fistula[edit | edit source]
Tracheoesophageal fistula is up to 90% associated with esophageal atresia. Clinical picture: soon after birth, mucus of the mouth and nose with foam, dyspnoea, cyanosis (due to aspiration of saliva or food). Diagnosis: insertion of the probe into the stomach (sometimes a contrast filling into the esophagus is required by the probe under X-ray control), aspiration pneumonia may be detected in the lung image. Therapy: surgical, intensive pre- and postoperative care. A fistula without esophageal atresia may be clinically silent at first and will manifest in older children because there is often a membrane or mucous plica in the mouth or the mouth is small and closes with compression when swallowed. Clinical picture: cough and cyanosis mainly during drinking, dilation of the stomach and intestines with air, aspiration pneumonia.
It occurs about 1:3000. It is caused by the abnormal separation of the trachea from the esophagus by the tracheoesophageal septum (it can also cause esophageal atresia). Mostly (90%) the upper part of the esophagus ends blindly, the lower one is connected by a fistula with the trachea. Isolated esophageal atresia and tr.e. fistula without esophageal atresia occurs less frequently (4%). Other defects are rare (less than 1%). The occurrence of these abnormalities is often associated with many other birth defects, such as heart defects. Polyhydramnios is sometimes a complication because fluids cannot pass freely through the digestive tract. Another possible complication is the passage of stomach contents into the trachea and lungs with subsequent inflammation.
Curiosity is VACTERL syndrome (Vertebral anomalies, Anal atresia, Cardiac defects, Tracheoesophageal fistula, Esophageal atresia, Renal anomalies, Limb defects). It is a set of congenital malformations that occur together much more frequently than would correspond to the random occurrence of each of them. However, the cause is unknown.
Congenital malformations of the lungs and bronchial tree[edit | edit source]
Large congenital abnormalities of the lungs and bronchial tree are relatively rare. Exceptionally, there is, for example, a blind-ending trachea with the absence of lungs, or agenesis of one lung.
However, abnormal branching of the bronchial tree is more common. This sometimes causes excessive lobes. However, these developmental abnormalities have little functional significance; the only complication may be during bronchoscopy.
The occurrence of ectopic lung lobes, which depart from the trachea or esophagus, is interesting. Ontogenetic, they arise from supernumerary laryngotracheal outgrowths of the front intestine, which forms itself independently on the main respiratory system.
Bronchomalacia[edit | edit source]
Bronchomalacia is congenital hypoplasia or the absence of cartilage rings. Clinical picture: may manifest as lobar emphysema in the neonate (but may be latent and manifest with frequent lung infections).
Special form: sy Williams-Campbell – congenital absence of cartilage from segmental bronchi onwards (trachea and bronchi are always OK).
Symptoms: chronic cough, dyspnoea, wheezing in inspiration and expiration, recurrent bronchopneumonia with purulent exudation. Diagnosis: X-ray – pulmonary hyperinflation and increased honeycomb diffusion bilaterally, bronchokinematography – shows generalized sac bronchiectasis with a noticeable change in the lumen depending on respiration. Therapy: administration of ATB, corticoids, mucolytics, and permanent therapeutic physical education (this will alleviate the intention).
Lung agenesis[edit | edit source]
Lung agenesis is a complete lack of bronchi, lung tissue, and vascular supply. Bilateral agenesis is rare (usually in anencephaly), more often is unilateral. Aplasia is more common – a rudimentary bronchus is formed. Clinical picture: unilateral may be well tolerated if it is not associated with other malformations, the other lung tends to be hypertrophic, herniation to the affected side. Diagnosis: on the X-ray there is a shadow of the hemithorax with a significant shift of the heart and mediastinum to the affected side and elevation of the diaphragm, asymmetry of the chest may manifest itself in the further development of the child, the diagnosis can be confirmed by bronchoscopy, ev. angiography. Therapy: prevention and consistent treatment of all respiratory infections
Pulmonary hypoplasia[edit | edit source]
Pulmonary hypoplasia is associated with hypoplasia of the bronchial tree (reduction in the number of branches) and the pulmonary system. It most often occurs in children with diaphragmatic herniation.
Pulmonary sequestration[edit | edit source]
Pulmonary sequestration is a mass of lung tissue that has no connection to the tracheobronchial tree and is supplied by an anomalous artery originating directly from the thoracic or abdominal aorta. There are two forms: extralobar sequestration – the sequestration has its own visceral pleura, often associated with other congenital malformations; and intralobar sequestration – it does not have its own visceral pleura. It is most often located in the posterobasal segment of the lower lobe. The sequestered lung is usually degenerated, fibrotic, inflammatory, or cystically altered. Clinical manifestation – in the first years of life it may manifest as a left-right short circuit, in older age as recurrent pneumonia. Diagnosis: X-ray – a sharply demarcated shadow resembling an infiltrate or atelectasis or the nature of a cyst or cysts. Therapy: surgical with a very good prognosis.
Congenital lung cysts[edit | edit source]
Congenital lung cysts belong to the most common lung anomalies. They are solitary or multiple, usually located in one lobe of the lung. Histologically, we distinguish between bronchogenic cysts, alveolar cysts, and combinations of both. Their size varies from microscopic to huge throughout the hemithorax.
They are caused by dilatation of the terminal bronchioles or larger bronchi. These cysts can be either small or multiple, which in the X-ray image determine the honeycomb appearance of the entire lung or one to two large cysts can occur. Cystic lung structures are usually poorly ventilated and are often the cause of chronic infections.
Clinical picture: communicating (tension) cyst can mimic pneumothorax with mediastinal deviation. Congenital cysts must be distinguished from postabscess pneumococci and lobar emphysema. The cyst has a typical hem. When left-sided, they can sometimes mimic a diaphragmatic hernia.
Therapy - extensive tension cysts manifest as an urgent condition requiring surgical treatment. Prognosis: secondary cyst infection with abscess, pyopneumothorax, bronchial fistula, and general sepsis is common. The risk of cyst malignancy is also reported.
Cystic adenomatoid lung malformation[edit | edit source]
Cystic adenomatoid lung malformation is a special and probably the most common form of the cystic lung. Excessive growth of terminal bronchioles and impaired alveolar differentiation. The cystic cavities communicate with the large bronchi, are lined with bronchial epithelium, and there are no cartilage and mucus glands in their wall. There is normal lung tissue between the cysts. Diagnosis: the affected part of the lungs is enlarged, there is a shadow on the X-ray (with various cystic brightnesses). Often associated with other congenital malformations, 50% of babies are born prematurely. Clinical picture: cyanosis and tachycardia soon after birth (with the onset of respiration, cysts expand and the surrounding healthy tissue is oppressed). Therapy: surgical removal of the affected lobe (often affecting only the segment, but resection of only the segment is not possible).
Congenital lobar emphysema[edit | edit source]
Congenital lobar emphysema is the opening of the pulmonary lobe by the mechanism of the bronchial valve closure. It most often affects one lobe (mainly the upper left). Causes: in the bronchial wall (cartilage anomalies,…), in the bronchial lumen (mucosal plicas), from external pressure (vessel, mediastinal cyst, or tumor). Clinical picture: progressive respiratory difficulties, tachypnoea, dyspnoea, cyanosis, cough, wheezing to stridor. The sooner this happens after birth, the greater the difficulties. The emphysema part compresses the surrounding parenchyma and deactivates it. Diagnosis: arch in the affected area, impaired breathing, and hypersonic percussion. X-ray: increased transparency of the affected lobe, fills almost the entire hemithorax, shifting the mediastinum. Differential diagnosis: foreign body aspiration, congenital tension cyst, emphysematous bulla, post-infectious pneumocele, diaphragmatic hernia.
Therapy: segment resection or lobectomy. Cases of spontaneous retreat are also described. We can administer oxygen (not overpressure – it would worsen the lobe opening), it is better to selectively intubate the main bronchus of a healthy lung.
Cardiovascular malformations causing pressure on the tracheobronchial tree[edit | edit source]
- The double aortic arch (complete vascular ring) – oppresses the trachea and esophagus – causes breathing and swallowing difficulties; basic examination: echocardiography, esophagogram,
- Incomplete vascular ring – consists of a right aortic arch with a left lig. arteriosum.
Insufficient surfactant[edit | edit source]
Surfactant deficiency (dysfunctional pneumocytes II, signaling pathway, etc.) causes respiratory distress syndrome – RDS (respiratory distress syndrome). In the absence of a surfactant, the surface tension on the alveolar fluid-air barrier rises, which causes a high risk of alveolar collapse during exhalation. The partially collapsed alveoli of the immature child contain a fluid with high protein content, with hyaline membranes and lamellar bodies apparently originating from the surfactant layer. RDS (formerly called hyaline membrane syndrome) is responsible for about 20% of newborn deaths. However, there are currently procedures for applying an artificial surfactant and inducing its production by glucocorticoids in preterm infants. This has enabled some children to survive from the middle of the sixth month of pregnancy.
Oligohydramnios and lung development[edit | edit source]
The presence of fluid in the lungs is an important stimulus for their development. If severe oligohydramnios (lack of amniotic fluid) is present in chronic form, lung development is delayed and severe pulmonary hypoplasia occurs.
Links[edit | edit source]
Source[edit | edit source]
- BENEŠ, Jiří. Studijní materiály [online]. [cit. 01.06.2009]. <http://jirben.wz.cz>.
Bibliography[edit | edit source]
- SADLER, Thomas W. Langmanova lékařská embryologie : Překlad 10. vydání. 1. edition. Grada Publishing, a.s
- MUNTAU, Ania. Intensivkurs Pädiatrie. - edition. Elsevier, Urban & Fischer, 2011. ISBN 9783437433931., 2011. ISBN 978-80-247-2640-3.
- MOORE, Keith L – PERSAUD, T.V.N. Zrození člověka. 1. edition. 2000. ISBN 80-85866-94-3.