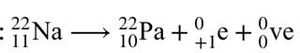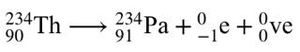Forum:Seminar papers/Biophysics/2. LF/2018-2019/Group 1/DEF
< Forum:Seminar papers | Biophysics | 2. LF | 2018-2019 | Group 1
Radioactivity[edit | edit source]
Introduction[edit | edit source]
Radioactivity is by definition “the property possessed by some elements or isotopes of spontaneously emitting energetic particles by the disintegration of their atomic nuclei.” (Ref: Merriam-Webster dictionary). It was first discovered in 1896 by Henry Becquerel, a French scientist.
Radioactive decay can be defined as the loss of energy by an unstable atomic nucleus, by emission of particles and /or photons. These can be α and β particles and/or γ photons.
In alpha decay, the parent radioisotope emits an alpha particle (identical to a helium nucleus, with 2 protons and 2 neutrons), decaying into a different atomic nucleus (since the number of protons decreased).
As for beta decay, there are two types: beta-plus decay and beta-minus decay. In beta-plus decay, a proton changes to a neutron, and the nucleus emits a positron and a neutrino.
In beta-minus decay, a neutron changes into a proton, and the nucleus emits an electron and an antineutrino.
Gamma decay is a process which can follow other types of decay and doesn’t involve a nuclear transmutation of isotopes (because the number of protons and neutrons of the radioisotope remains the same). A high-energy nucleus releases gamma ray photons and is brought to a lower-energy state.
Clinical importance[edit | edit source]
The world has benefited from the discovery of radioactivity, especially in the fields of nuclear medicine imaging and cancer treatment.
The main significance of nuclear medicine imaging is for diagnostic investigation. Images we can get from different techniques help to diagnose and evaluate patient pathologies. Major techniques are the well known Scintigraphy, SPECT and PET scans. An advantage of these nuclear medical images is that they enable the depiction of physiological processes in the body by using radioactive tracers, that generate radioactive decay.
Radioactivity can also be used in cancer treatments (part of nuclear medicine therapy or in brachytherapy sources). The high energy radiation kills or interferes with the tumour cells, damaging their ability to proliferate. One of the disadvantages is that surrounding healthy cells also can be irreversibly damage.
Literature review[edit | edit source]
Radioactivity has brought about a revolution in the medical field during the last century and is still an essential tool today in both diagnostic investigation and therapy. Nuclear medicine tests are minimally invasive procedures, that allow medical personnel to diagnose, treat or monitor health conditions, diseases and physical traumas of patients.
In nuclear medicine, radiation is administered, making the patient radioactive for a short period of time. Various vital processes like organ function, blood flow, infection, and many others are monitored. Positron-emission tomography (PET) scan uses a positron emitting radioactive tracer and generates images with a computer to detect cancers and memory disorders. In Brachytherapy, a small radioactive material is implanted in/near a cancerous tumour to expose it to a constant stream of radiation.
The risks or the disadvantages associated with the use of ionizing radiation like radioactive materials in the medical field is that a person can develop DNA mutations or second cancer later in life. Major ethical problems in nuclear medicine are the justification of medical exposures in practice and intentional non-medical human exposure, as well as the use of radiation on children and pregnant women. Reports in Radiology Journal have reported that there is a significant level of inappropriate usage and a poor level of awareness of dose and risk in some practice, including non-compliance with the law.
Equipment[edit | edit source]
BetaGamma kit:
- Radiation sensor
- Beta and Gamma radiation source
- Absorbing plates: Iron \ Copper \ Aluminium
- Arresting pin
- Tripod holder
- Digital pulse counter
Excel spreadsheet
Techniques: Step-by-step[edit | edit source]
- Set the BetaGamma kit up by putting the Beta/Gamma radiation detector on the tripod holder
- Use the connecting cord to connect the digital pulse counter to the β/γ radiation detector and switch on both devices
- In order to assess the background radiation of the environment that you will be working on, make sure that there are no radioactive materials nearby. Therefore place the radioactive source in another room, because it will interfere with the measurements.
- Measure the background radiation for 100 seconds by pressing the ‘100s’ button on the digital pulse counter repeat 10 times and write it down on excel table. (little tip: make sure you write down on an actual piece of paper as well. You might have to redo it because of technical inconveniences)
- You can now bring the radiation source back and place it 4 cm from the β/γ radiation indicator (first gap closest to the indicator). Enter the arresting pin into the gap on the tripod holder perpendicular to the radiation source.
PROTECTION BY DISTANCE[edit | edit source]
- In order to detect the beta particles, make sure that the open mouth of the radiation detector is facing the side labeled ‘B’ on the head of the source.
- Start by pressing the ‘10s’ button on the pulse counter and repeat 5 times to obtain an average value.
- Repeat the procedure that you did in step 5 to 7 again but increase the distance of the radiation source and the arresting pin to 8 cm (second gap away from the β/γ radiation detector) and then increase it to 12cm and 16cm (3rd and 4th gap). Take 5 measurements at each one of the distances
- You must repeat steps 5 to 8 with gamma radiation, for gamma photons, use the opening that is on the side where its labeled as ‘G’ on the head.
PROTECTION BY SHIELDING[edit | edit source]
- Place the radiation source back in the first away gap. Place an aluminium (Al) absorption material sheet between the two components on the mortise slit of the tripod holder
- Take 5 measurements for beta particles in each gap and repeat for gamma particles and determine an average (excel)
- Do the step 10-11 again for both Iron (Fe) and Copper (Cu). Again, start in the closest gap and measuring 5 times each gap (10s) move the source further away. Do this for both radiations as well.
- Enter the data collected into an Excel spreadsheet template. This will help you prevent errors in calculation and can automatically create graphs that you need for comparison of the types of radiation and their correlations
- Calculate transmittance and absorbance for each material
- Calculate the attenuation coefficient and HVL (The Half Value Layer is the thickness of any given material which stops 50% of the incident particles/photons).
Conclusion[edit | edit source]
From the data and knowledge, that can be obtained by completing this biophysical experiment, the effect of radioactivity as well the impact of absorption layers and distance can be deducted. This enables reevaluation of the information about radioactivity. Experiments like this help understand how regularly exposed patients and doctors can be protected and how to correctly work with radioactive material. You can see the effects of this type of research in a clinical nuclear medicine room, where not only lead protections are worn, but also the assistants go into separate rooms to increase distance. Since this lead protections can actually become radioactive them-selves owing to contamination they should be checked regularly and decontaminated. In conclusion, the potential for the clinical application of radioactivity is great and for sure there will be more scientific development in this field to further medical possibilities and to work in the field in a safe manner.
References[edit | edit source]
Nagasaki University. Radiation Q and A. https://www-sdc.med.nagasaki-u.ac.jp/abdi/publicity/index_e.html
Malone J. (2013). Radioactivity in the Environment - Ethical Issues. Clinical Radiology, Volume 19. https://www.sciencedirect.com/science/article/pii/B9780080450155000071
Radiation in Medicine: Medical Imaging Procedures https://www.cdc.gov/features/medical-imaging-procedures/index.html
Malone J. (2012). Justification of diagnostic medical exposures. British Journal of Radiology. 85:523-538; https://www.birpublications.org/loi/bjr