Hybridization (chemistry)
Hybridization of orbitals is a phenomenon of energetic unification of energetically different orbitals of a given atom. Hybridization takes place in those orbitals of the atom that provide their electrons to form covalent bonds. The concept of hybridization of orbitals is very useful for explaining the shape of molecules. For each type of hybridization, there is a characteristic distribution of hybrid orbitals in space, which also determines the spatial arrangement of chemical bonds.
| A kind of hybridization | Geometry of the molecule |
|---|---|
| sp | linear |
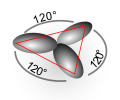
| sp2 | equilateral triangle |
| sp3 | tetrahedron |
| d2sp3 | octahedron |
| dsp2 | square |
| dsp3 | trigonal bipyramid or square pyramid |
- Spatial arrangement of hybridized orbitals
By hybridization, a set of atomic orbitals with different energy is formed into a set of orbitals with the same energy. This achieves the equivalence of bonds between the central metal ion and the ligand. The geometric structure of the complex shown by the coordination polyhedron, as the vertices are directed to bonds, corresponds to the minimum energy of the system.
The number of ligands bound to the central atom (ion) is equal to the coordination number, which is the same as the number of orbitals participating in the hybridization. When creating and filling hybrid orbitals, the central particle aims to reach the configuration of the outer layer of atoms of the nearest next noble gas in the PSP (Periodic Table of Elements) if possible.
The box diagrams of the complexes [Fe(H2O)6]3+, [ Ni(CN)4]2- and [Ag(NH3)2 ]+.
In the following table we find the common types of hybridization, the number and arrangement of ligands (coordination polyhedra) as well as several examples of the respective complexes.
You can get a good idea about orbital hybridization from the following video:
<mediaplayer>https://www.youtube.com/watch?v=g1fGXDRxS6k</mediaplayer>