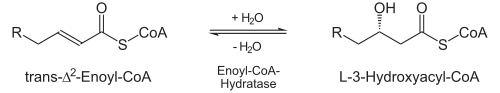Introduction to lipid breakdown and ketone body metabolism
Triacylglycerols (TAGs) store large amounts of chemical energy. As an energy store, they are very advantageous because 1 g of anhydrous TAG stores six times more energy than 1 g of hydrated glycogen. The complete oxidation of 1 g of TAG yields approximately 38 kJ, while only 17 kJ are obtained from 1 g of carbohydrates or proteins. A 70 kg man stores approximately 400,000 kJ in his TAGs - the total weight of a TAG is around 10.5 kg. These supplies could allow us to survive several weeks of starvation. The main site of TAG accumulation is the cytoplasm of adipocytes.
Fatty acid oxidation[edit | edit source]
Individual types of fatty acid oxidation are indicated by Greek letters, which determine the carbon atom on which the reactions take place. β-oxidation taking place in the mitochondrial matrix is of major importance. Enzymes catalyzing the so-called ω- and α-oxidation occur on the membranes of the endoplasmic reticulum.
Conversion of fatty acids to glucose[edit | edit source]
Animals cannot convert fatty acids into glucose. Fatty acids represent a rich source of energy for gluconeogenesis, but glucose is not formed from their carbon atoms (with the exception of fatty acids with an odd number of C). Acetyl-CoA cannot be converted to either pyruvate or oxaloacetate - both carbons are split off as CO2 during the Krebs cycle. The pyruvate dehydrogenase reaction is irreversible. Interestingly, plants also have two other enzymes that allow them to convert AcCoA to OAA, in the so-called glyoxylate cycle.