Markers of muscle tissue damage, significance and determination
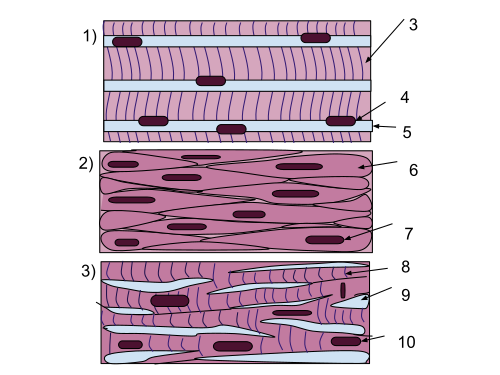
Structure of muscle tissue[edit | edit source]
Muscle tissue is a fundamental component of the human body, responsible for movement, stability, and essential physiological functions. There are three main types of muscle tissue:
- Skeletal muscle – Voluntary, striated muscle attached to bones, allowing body movement through contraction.
- Smooth muscle – Involuntary, non-striated muscle found in internal organs (e.g., blood vessels, digestive tract), controlling automatic functions like digestion and circulation.
- Cardiac muscle – Involuntary, striated muscle found only in the heart, specialized for continuous rhythmic contractions.
Muscle fibers, also known as myocytes, contain one or more centrally located oval nuclei. In close proximity to the nuclei, numerous mitochondria and glycogen granules are present, ensuring an energy supply for contraction. The cytoplasm of muscle cells (sarcoplasm) also contains myoglobin, which facilitates oxygen storage. The sarcoplasmic reticulum, consisting of a system of vesicles and cisternae, serves as a reservoir for calcium ions (Ca²⁺), which are essential for muscle contraction.
Muscle fibers are composed of two main types of myofilaments:
- Thick (myosin) filaments – These are formed by myosin molecules, which have both a fibrillar and a globular part, where ATPase activity is located.
- Thin (actin) filaments – These consist of filaments arranged in a double helical structure, formed by the polymerization of globular actin monomers.
These structures enables the contraction mechanism of muscle tissue, allowing movement and force generation in various parts of the body.
Muscle damage[edit | edit source]
Muscle damage can occur due to various causes, ranging from mechanical injury to metabolic disturbances and systemic diseases. Primary causes include:
- Mechanical factors: trauma, injury and overuse
- Ischemia : vascular occlusion, thrombosis, infarction
- Inflammations
- Metabolic disease
- Endocrine imbalances: hypo/ hyperthyroidism, Cushing's syndrome
- Drug induced
Markers of muscle damage[edit | edit source]
- Myoglobin
- Creatine kinase
- LD
- AST
- Troponin
Myoglobin[edit | edit source]
Myoglobin is a globular protein composed of a single polypeptide chain that contains heme as a prosthetic group. It reversibly binds and transports oxygen within muscle cells. Myoglobin found in both skeletal muscle and the myocardium is identical and serves as an oxygen reservoir for these tissues. In the kidneys, it is filtered by the glomerular membrane and excreted in urine. Its biological half-life is very short, ranging from 10 to 20 minutes.
Unlike hemoglobin, myoglobin contains only one heme group and a single globin chain, allowing it to transport just one molecule of O₂. However, its affinity for oxygen is significantly higher than that of hemoglobin.
As a cytoplasmic protein with low molecular weight, myoglobin is rapidly released from damaged tissue. Myoglobin is considered one of the most sensitive biochemical markers for early detection of muscle damage. However, its major limitation is its lack of specificity. Elevated myoglobin levels can also be observed in:
- Any skeletal muscle injury, including intramuscular injections or minor trauma from falls.
- Intense physical exertion, such as excessive abdominal strain during prolonged vomiting.
- Renal insufficiency, which impairs myoglobin clearance.
Creatine kinase[edit | edit source]
Creatine Kinase is predominantly found in skeletal muscle, myocardium, and brain tissue. It is composed of two subunits, which exist in two types:
- M (muscle) subunit
- B (brain) subunit
Different combinations of these subunits form the three creatine kinase isoenzymes:
- CK-BB (CK-1) – Brain isoenzyme
- CK-MB (CK-2) – Myocardial isoenzyme
- CK-MM (CK-3) – Skeletal muscle isoenzyme
In skeletal muscle, CK-MM predominates, but CK-MB is also present in smaller amounts. In the brain, CK-BB is found, but it does not cross the blood-brain barrier under normal conditions. The myocardium primarily expresses CK-MB, although CK-MM is also present. CK levels can be influenced by factors such as age, sex, muscle mass, and physical activity.
CK-MB has greater diagnostic value than total CK. Elevated CK-MB levels may also result from:
- Skeletal muscle damage (trauma, muscular dystrophies, intramuscular injections, CPR, defibrillation)
- Extreme physical exertion
- Chronic renal insufficiency
CK-MB can be measured in two ways:
- Enzymatic activity assay, detecting only active enzyme molecules
- Immunochemical mass concentration assay (CK-MB mass), which detects both active and partially degraded molecules that have lost enzymatic function
Lactate dehydrogenase[edit | edit source]
Lactate Dehydrogenase (LD or LDH) is an oxidoreductase enzyme that catalyzes the reversible conversion of lactate to pyruvate.
The enzyme consists of four subunits, each with a relative molecular weight of 34,000. It exists in five isoenzymes, classified as:
- LD1 (H₄)
- LD2 (H₃M₁)
- LD3 (H₂M₂)
- LD4 (H₁M₃)
- LD5 (M₄)
LDH is found in the cytoplasm of cells in many tissues. It is released into circulation even with mild tissue damage, making it a useful marker for cell injury and disease monitoring.
Aspartate aminotransferase[edit | edit source]
AST is an important biomarker for tissue damage. It is typically measured in blood tests to assess liver function, muscle injury, and myocardial infarction.
1. AST in Liver Diseases[edit | edit source]
AST is often elevated in liver diseases, but it is not liver-specific. It is usually interpreted in conjunction with alanine aminotransferase (ALT):
- AST > ALT (AST/ALT ratio > 2): Suggestive of alcoholic liver disease.
- ALT > AST: Suggestive of non-alcoholic fatty liver disease (NAFLD) or viral hepatitis.
- AST/ALT ratio near 1: Can be seen in chronic liver disease or cirrhosis.
2. AST in Myocardial Infarction (Heart Attack)[edit | edit source]
- Historically, AST was one of the first biochemical markers used for diagnosing acute myocardial infarction (AMI).
- Rise in AST occurs 6–12 hours after myocardial injury, peaks at 24–48 hours, and normalizes within 4–6 days.
- AST is not cardiac-specific, and modern markers like troponins (TnT, TnI) and CK-MB have replaced AST in diagnosing myocardial infarction.
3. AST in Muscle Disorders[edit | edit source]
- Increased AST levels are found in muscular dystrophy, rhabdomyolysis, polymyositis, and trauma-related muscle injury.
- Since AST is present in both skeletal muscle and the heart, it is not specific for differentiating between cardiac and muscular injury.
4. AST in Hemolysis and Kidney Diseases[edit | edit source]
- AST is present in red blood cells, hemolysis can cause a mild increase in AST levels.
- Chronic kidney disease (CKD) may also result in mildly elevated AST due to reduced clearance.
Troponin[edit | edit source]
Troponin T (TnT) and Troponin I (TnI) are used as cardiac biomarkers. These proteins are found in both skeletal muscle and myocardium, but their cardiac isoforms (cTnT and cTnI) have a unique amino acid composition, making them specific for the myocardium. The majority of cardiac isoforms of TnT and TnI are bound within the contractile apparatus and are released into circulation due to proteolytic degradation.
Troponins are measured using high-sensitivity immunochemical assays, which allow for early detection of myocardial injury.
Reference[edit | edit source]
Markers in Acute Coronary Syndrome [1]
Biochemická vyšetření u akutního infarktu myocardu [2]