The cell cycle, its regulation and disturbances
The eukaryotic cell cycle is also known as the “cell division cycle”. It is a process whereby a mother cell will give rise to two identical daughter cells.
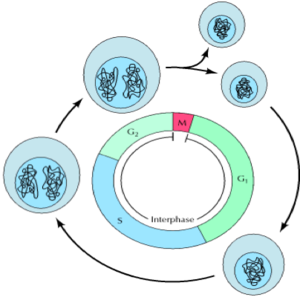
The cell cycle has 4 discrete phases: 1) M-phase: stages of mitosis and cytokinesis. 2) G1 (gap phase 1): interval between mitosis and DNA replication. The cell is metabolically active and grows, but DNA replication does not occur. 3) S-phase: DNA replication occurs. 4) G2 (gap phase 2): Continuation of cell growth and synthesis of proteins in preparation for mitosis.
Cells at different stages of the cell cycle can be distinguished by their DNA content. Cell in G1 contain two copies of each chromosome, so their DNA content is referred to as 2n (diploid). DNA replication occurs during S phase, so DNA content of the cell is increased from 2n to 4n. DNA content remains at 4n for cells in G2 and M, decreasing to 2n after cytokinesis.
Events of the cell cycle, as seen under the microscope, include mitosis and cytokinesis (otherwise known as M-phase). M-phase occupies a small fraction of the cell cycle and involves the separation of daughter chromosomes and cell division. The remaining longer part of the cell cycle is known as interphase. In this phase, chromosomes are condensed and become evenly distributed throughout the nucleus in preparation for replication and eventually cell division (mitosis).
Stages of mitosis include prophase, prometaphase, metaphase, anaphase and telophase which correspond to the separation and movement of chromosomal DNA in a cell during cell division. Cytokinesis is the mechanical action of the mother cell splitting, giving rise to two daughter cells. Each cell then enters interphase, whereby cell growth and DNA replication occur ready for further cell division.
Cell cycle control:
Events of the cell cycle and tightly controlled at the point of entry into the cell cycle and at several important check points.
Entry into the cell cycle is controlled by growth factors which signal cellular proliferation at a point in late G1 called the restriction point. If growth factors are not available to the cell in G1, the cell enters a quiescent stage called G0. Cells are metabolically active in G0 but growth ceases and protein synthesis in the cell is reduced. A cell can exit G0 when stimulated by extracellular signals.
Cell cycle check points function to ensure that complete, correct genomes are transferred to daughter cells. This control is exerted by proteins called cyclin-dependent kinases (Cdks). These proteins phosphorylate (add phosphate groups) their targets which go on to initiate various events of the cell cycle.
Cdks themselves are regulated by molecules called cyclins. The availability of cyclins controls the activity of Cdks and promotes cycle progression e.g. when complexed with M-phase cyclin, the cdk triggers the mitosis machinery. When complexed with S-phase cyclin, the cdk triggers DNA replication.
G1 checkpoint: allows repair of DNA damage to take place before the cell enters S phase, where the damaged DNA would be replicated. Arrest at the G1 checkpoint is mediated by the action of a protein transcription factor known as p53. Fact: the gene encoding p53 is frequently mutated in human cancers. Loss of p53 function leads to the replication of damaged DNA and inheritance into daughter cells. This increases the frequency of mutations and instability of the cellular genetics, contributing to cancer development.
G2 checkpoint: prevents initiation of mitosis until DNA replication is completed. If un-replicated or incomplete DNA is sensed at this check point, cell cycle arrest occurs.
Metaphase checkpoint: maintains the integrity of the genome occurs toward the end of mitosis by monitoring the alignment of chromosomes on the mitotic spindle. This guarantees that a complete set of chromosomes is distributed correctly to the daughter cells.
Disturbances to the cell cycle:
Disturbances to the cell cycle may occur by disruption to: - Positive cell cycle regulators: proto-oncogenes - Negative cell cycle regulators: tumour suppressor genes
Many oncogenes and defective tumor-suppressor genes promote malignant change by stimulating cell-cycle entry, or disrupting the checkpoint response to DNA damage.
Proto-oncogenes are normal genes that cause a cell to become cancerous when mutated. For example, mutations to Cdk proteins could over-ride a cell cycle checkpoint without meeting the required conditions. This may lead to the production of atypical daughter cells that are able to divide further and accumulate more mutations.
Tumor suppressor genes are negative regulator proteins that prevent the cell from uncontrolled cell division. These include retinoblastoma protein (RB1), p53, and p21, which block cell-cycle progress until certain requirements are met. A cell that carries a mutated negative regulator might not be able to cause cell cycle arrest if there is a problem.