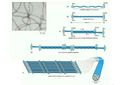
Types of cytoskeletal filaments
Introduction[edit | edit source]
Although there are different types, all cellular cytoskeletons have thin, fibrous structures and are polymers of basic unit proteins. They have unique characteristics and perform various functions according to their nature.
The basic functions of the cytoskeleton are the formation of a structural shaping frame of the cell, the guidance in moving of the cell in its environment and of the cellular interior and furthermore the cell signalization.
The roles of the cytoskeleton can be organized in both structural and functional ones. The main structural roles of the filaments in a cell are the preservation of its shape and the organisation and arrangement of its various organelles. The functional roles are played through the interaction with other proteins, e.g. muscle contraction, cell locomotion, cell division and intracellular transport.
The three types of cytoskeletal filaments are:
- Microtubules
- Intermediate filaments
- Microfilaments (actin filaments)
Mechanical properties of individual types of cytoskeletal fibres
Microtubules:[edit | edit source]
Microtubules can be found within the cytoplasm of all eukaryotic cells. They have a ring-shaped structure, with an (outer) diameter of 25 nm and vary in length. This type of cytoskeletal filaments is synthesised by the polymerization of a heterodimer of two globular proteins, the α and β tubulin.
Structure:[edit | edit source]
Microtubule: 13 protofilaments protofilaments: polymer consisting of dimers of α and β tubulin
Polymerization: binding of GTP (GDP) + end, - end of microtubules
- dynamic instability
MTOC (microtubules organizing center)
- structure found in eukaryotic cells from which microtubules emerge
- two main functions:
- organization of eukaryotic flagella and cilia
- organization of the mitotic and meiotic spindle apparatus (which separate the chromosomes during cell division)
Function:[edit | edit source]
Mitotic spindle: centrosomes
Flagella and cilia: structure (9 doublets +2) movement (motor protein dynein)
Tracks for the movement of organelles: motor proteins (molecular motors) dynein a kinesin
Intermediate filaments:[edit | edit source]
Intermediate filaments can be found in the cytoskeleton of many eukaryotic cells. They have an average diameter of 10 nm, which is between the average diameter of the micro-/ actin filaments (7 nm) and that of the microtubules (25 nm). They are composed of various related proteins that share similar structures but that perform in different types of cells. More than 50 different proteins of intermediate filaments can be distinguished. Based on the heterogeneity of those proteins, the intermediate filaments that are formed consequently have been classified into six subgroups. The classification is based on identified similarities and differences between the varying amino acid sequences of the different proteins.
Type I and II[edit | edit source]
These filaments are formed of two groups of acidic (I) and basic (II) cytokeratins (each consists of about 15 different proteins), which contribute to specific junctions between epithelial cells. In order to form a filament, each type of epithelial cells has to synthesize at least one type I and one type II, which then copolymerize. The keratins produce filaments that are, depending on their distinctive chemical and immunological properties, of basic importance for several diverging cellular functions. The evolution of keratins made living in a terrestrial ecosystem possible, e.g. due to a differentiation process of epidermal cells that resulted in a reduction of harmful dehydration. A disruption of the acidic cytokeratin can cause some blistering skin disease, a disruption of the basic cytokeratin can cause corneal dystrophy and keratoderma (abnormal thickening of the palms and soles).
Type III[edit | edit source]
Type III includes various types of proteins that are found in different kinds of cells. The protein desmin contributes to muscle cells, vimentin is expressed in mesenchymal cells (white blood cells, fibroblasts and smooth muscle cells). Other specialised proteins of the type III are Synemin, which is also distributed in muscle cells, peripherin, which is expressed in neurons (especially of the peripheral nervous system) and the protein GFAP, which can be found in astrocytes (glial cells). A disruption of the protein desmin is involved in the development of myopathies.
Type IV[edit | edit source]
This type includes the protein α-internexin, which is expressed at an early stage of the embryonic neuron development and the three neurofilament proteins NF-L, NF-M and NF-H, which play important roles in many types of mature neurons.
Type V[edit | edit source]
The type V proteins, the nuclear lamins, are expressed in the nuclei of all eukaryotic cells. Their function and structure differ from those of the other intermediate filament proteins. If the protein lamins is disrupted in any way, it distributes to the development of cardiomyopathy, muscular dystrophies and infrequently the progeria syndrome (genetic disorder with symptoms of aging at very a young age)
Type VI[edit | edit source]
Type VI of intermediate filament proteins includes the protein nestin, which is expressed in some stem and embryonic cells.
Microfilaments:[edit | edit source]
The monomers of the microfilaments are composed of actin, which is why this type of cytoskeletal filament is also known as ´actin filaments´. Their average diameter is between 5-7 nm.
Structure:[edit | edit source]
Fibres: polymers of actin
- The basic unit of actin filaments is a protein called G-actin (which has a similar structure to many organisms such as ameba, plants and humans).
Microfilaments: double-helix
- G-actin polymerizes to form actin filaments with a diameter of around 7 nm.
Polymerization: binding of ATP (ADP) + end, - end of microfilaments -Dynamic instability
- Since G-actin molecule has plus end and minus end, the polymerized filament also has plus end and minus end.
- G-actin has a binding site for ATP or ADP, and ATP bound G-actin molecules polymerize stably. However, after polymerization, when bound ATP is hydrolyzed into ADP, the polymer will become unstable and can easily depolymerize. After depolymerization, when ADP is replaced with ATP, G-actin molecules again become able to bind to actin filaments.
Function:[edit | edit source]
Microvilli
- in cell processes, they are involved in the formation of the pseudopodia (processes) of moving cells and processes known as microvilli often found in usual cells
Cell cortex: structure and localization function
- lamellipodia, filopodia, pseudopodia: amoeboid locomotion of the cell
- when located directly below the plasma membrane; the actin filament is stabilized and tethers the membrane proteins by forming a network structure
Contractile ring: cytokinesis
- actin filaments are especially abundant in the contractile apparatus of muscle cells.
- contractile bundles: “muscles” of the cell
- associated with motor protein myosin
References:[edit | edit source]
- ↑ Alberts B. et al.: Essential Cell Biology. Garland Science. New York and London, pp. 571-607, 2010.
- ↑ Anthony L. Meschler: Junqueiras Basic Histology Text & Atlas, 13th edition, McGrawHill Lange, ISBN 978-0-07-180798-2
- ↑ University of Tokyo: Life Science Textbook, Komaba Organization for Educational Excellence, pp. 109-123, 2010