Colorectal Cancer/PGS
Colorectal cancer (CRC) is one of the most common cancers in developed countries. About a third of patients die as a result of cancer, but it is still one of the most common causes of cancer-related deaths worldwide. It can arise on the basis of polyps, a smaller number of tumors can also arise without previous polyps. It is reported that up to 85% of colorectal cancer cases arise sporadically under the influence of risk factors, especially lifestyle, the rest of the tumors are of hereditary origin. The actual proportion of hereditary influences is probably much higher, e.g. milder forms of Lynch syndrome often escape diagnosis. The basis of therapy is surgery, and the basis of therapeutic success is early diagnosis, usually thanks to screening during the period when the tumor is clinically silent. Colorectal cancer can occur in several ways, the most common, originally considered to be the only one, is the way of mutation of the APC gene with subsequent alteration of the Wnt signaling cascade.
History[edit | edit source]
There is no reliable information that colorectal cancer was present even in prehistoric times. However, this cannot be considered as proof that colorectal cancer or other tumors do not occur. In reality, soft tissues are only rarely preserved as a source for research in the field of historical anthropology or even paleontology. Even metastatic bones are more susceptible to faster decomposition, so surviving traces of metastatic skeletal involvement is relatively rare.[1]
Sources from historical periods are greatly complicated by the fact that the basic tool of doctors of different historical eras was external description. Tumor disease in today's sense of the word was not usually defined, it could be classified between swellings together with abscesses or between ulcerations together with some infections. From antiquity to the Middle Ages, Galen's theory persisted, according to which a tumor is a local congestion of black bile. [1]Only the works of A. Vesalius (1514-1564) and G. B. Morgagni (1681-1771) with a thorough description of tumors questioned the humoral theory and created space for new speculative theories, e.g. Paracelsus' (1493-1541) theory about the stagnation of mineral substances from the external environment or Le Dran's (1685-1770) theory on the lymphogenic origin and spread of tumors. Confusion about the origin of tumors has been associated with a number of speculative approaches to treatment. The speculative nature of theories about the nature of tumors did not prevent even modern doctors from using relatively drastic and dangerous procedures consisting, for example, in the application of toxic substances, such as mercury. In response to the results of such therapeutic efforts, the opposite extremes, consisting in the administration of biologically practically inert substances, were understandably gaining popularity.[2] Even, despite generally unsuccessful interventions, there were sometimes rather sharp clashes of opinions between supporters of different theories and therapeutic procedures, including public promotion of their approach and attacking the opposing party. The clash between proponents of (and not only) mercury venereal disease therapy and proponents of therapy with plant juices and extracts is relatively well-known.[3][2]
The discovery that tumors are cellular is associated with the work of J.P. Müller (1801 – 28 April 1858) and Th. Schwann (1810-1882) from the end of the first half of the 19th century. The theory describing the concepts still used today, initiation, growth, local spread and metastatic spread, was formulated in 1865 by C. Thiersch (1822-1895), H.W.G. von Waldayer-Hartz (1836-1921) supported this therapy with strong evidence.[2]
The earliest probable description of colorectal cancer in medieval Europe appears in Irish texts from the 9th century and Saxon texts from the 10th century. The description seems to correspond to a tumorous obstruction of the large intestine.[2]
One of the oldest surviving descriptions from medieval Europe, which certainly captures colorectal cancer, including a description of the tumor itself and its manifestations and prognosis, was left by the English surgeon John of Arden in the 14th century. Among other things, the author mentions that he saw many who died of the disease. However, he had never seen or heard of anyone who was cured of the disease.[2]
The first therapy with hope for therapeutic success was a radical surgical resection of the affected section of the intestine. The first successful resection of a rectal tumor was performed in 1829 by J. Lisfranc (1790-1847). Rectal cancer surgery did not become more widespread until the beginning of the 20th century, mainly due to a significant reduction in postoperative mortality. Even in the smaller part of patients who underwent the procedure, approximately 90% of patients relapsed. With the gradual improvement of the technique and the extent of the procedure, in the 1930s the perioperative mortality was already 6% and the five-year survival increased to 65%.[2] In 1954, W.H. Cole published work showing that tumor cells could appear in the portal blood of an operated patient during surgery; this discovery gradually led to the introduction of the "no touch" concept in surgical procedures on the colon and rectum.[4] Work safely demonstrating that extending the procedure to mesorectal fat improves patient survival was not published until 1986 by RJ Heald and JD Ryall.[2]
The discovery of X-rays and radioactivity, and especially the finding that ionizing radiation inhibits cell growth, led to the use of these modalities as supportive therapy already at the beginning of the 20th century. However, the original methods of application did not significantly affect the survival of patients.[2]
The finding that mustard gas can inhibit hematological malignancies also led to attempts to treat colorectal cancer with substances derived from mustard gas (the first alkylating cytostatics). The results of these experiments were not encouraging, colorectal cancer is only slightly sensitive to the effects of alkylating cytostatics. In 1957, fluorouracil was introduced as a representative of a new class of cytostatics (antimetabolite), which proved to be very effective in the therapy of colorectal cancer.[2]
Epidemiology[edit | edit source]

Extension[edit | edit source]
It is estimated that more than one million new patients appear worldwide each year, and approximately one third of patients die as a result of the disease.[5] According to analyzes of data from the turn of the 19th and 20th centuries, untreated colorectal cancer ends in death on average within two years of the onset of symptoms, within two years without anticancer treatment, with the exception of palliative care, more than 60% of patients died; exceptionally, the survival of the patient for many years has also been observed.[6][7] By comparing the current results, it can be clearly stated that the prognosis of treated patients is significantly better than that of patients without oncological therapy.
Together with Slovakia, Hungary, Israel and New Zealand, the Czech Republic belongs to the countries with the highest incidence of colorectal cancer.[8]
Age-standardized incidence and mortality is according to GLOBOSCAN 2012[9]
| sex | age-standardized incidence (per 100,000) | age-standardized mortality |
|---|---|---|
| Men | 54,0 | 22,6 |
| Women | 27,1 | 9,9 |
| Both Sexes | 38,9 | 15,4 |
These data refer to an age-standardized population, enabling international comparisons between populations with different age structures. In 2011, the actual incidence of colorectal cancer in the Czech Republic was 38.36 cases per 100 thousand, and mortality 16.46 per 100 thousand. resident.[10] Even if the incidence has an increasing trend, the mortality is relatively stable in the long term. The representation of individual clinical stages does not change much over time, so stable mortality with increasing incidence is more indicative of the increasing availability and quality of medical care.[8]
Risk Factors[edit | edit source]
Most cases of colorectal cancer occur sporadically. The main risk factors are:[5]
- Demographic factors:
- Age,
- Male gender.
- Environmental factors:
- Intake of red meat,
- High-fat diet,
- Sedentary lifestyle
- Smoking,
- Alcohol.
- Diseases in the anamnesis:
- History of colorectal polyps,
- History of colorectal cancer,
- Diabetes mellitus,
- Idiopathic intestinal inflammations.
Genetic Syndromes[edit | edit source]
Some genetic syndromes may be associated with a greater or lesser risk of developing colorectal cancer. The age of manifestation may vary. While, for example, familial adenomatous polyposis causes colorectal cancer at a young age, milder forms of Lynch syndrome can only lead to colorectal cancer at a relatively old age.
The most common genetic syndromes associated with colorectal cancer:
- Familial adenomatous polyposis and its variants,
- Lynch Syndrome,
- Peutz-Jeghers syndrome,
- Juvenile Polyposis.
Pathology[edit | edit source]
Molecular Pathology[edit | edit source]
From a molecular point of view, colorectal cancer represents a group of several different diseases with some overlap. Malignant reversal can occur as a result of several sequences of mutations. Based on molecular characteristics, five types of colorectal cancer can be distinguished. Individual types are distinguished according to hypermethylation of CpG islands (CIMP), microsatellite instability (MSI, MSS = stable), chromosomal aberrations and a characteristic mutation:[11]
- Typ 1 CIMP-H, MSI-H, BRAF mutation, chromosomes stable. It represents about 12% of colorectal cancers, it is also referred to as sporadic MSI-H colorectal cancer. It arises on the basis of serous lesions.
- Typ 2 CIMP-H, MSI-L nebo MSS, BRAF mutation, chromosomes stable. It represents about 8% of colorectal cancers, it arises on the basis of serous lesions.
- Typ 3 CIMP-L, MSI-L nebo MSS, KRAS mutation, chromosomal instability. It accounts for about 20% of colorectal cancers. It arises on the basis of serous lesions and classic adenomas.
- Typ 4 CIMP-negative, MSS, chromosomes unstable. It arises on the basis of congenital or acquired APC or MUTYH, it arises from classical adenoma. It accounts for about 57% of colorectal cancers.
- Typ 5 CIMP-negative, MSI-H, chromosomes are stable. It arises on the basis Lynch syndrome, sometimes referred to as familial MSI-H carcinoma. It accounts for about 3% of colorectal cancers.
In sporadic colorectal cancers, three large circuits of changes in cell biology can be distinguished, we can speak of three pathways:[12]
- Chromosomal instability pathway,
- Microsatellite instability pathway,
- the CpG island hypermethylation pathway.
The chromosomal instability pathway[edit | edit source]
Chromosomal instability is the most common cause of genetic instability in colorectal cancers, it can be demonstrated in 65-70% of all cases. It includes a wide range of chromosomal changes, the consequence of which can be both the amplification of some genes and the loss of heterozygosity of other genes. The following changes occur most often in the following locations:
- Extensive gain: 7, 8q, 13q, 20, X.
- Extensive loss: 1, 4, 5, 8p, 14q, 15q, 17p, 18, 20p, 22q.
- Focal gain/loss of genes: VEGF, MYC, MET, LYN, PTEN.
- Multiple allele losses: 1, 5, 8, 17, 18.
- Loss of an entire chromosome: 18.
The most significant molecular changes at the level of a single gene are mutations of APC, MCC and K-ras, at the level of larger regions of the tail on 5q, 8p, 17p and 18q.[12]
- APC
See the APC page for more detailed information
APC is a tumor suppressor gene whose congenital mutation is responsible for most cases of familial adenomatous polyposis. The product of the gene is a multifunctional 310 kDa protein involved in the homeostasis of epithelia in particular by regulating the degeneration of cytoplasmic β-catenin and is thus a key part of thewnt signaling cascade. APC is also involved in cell cycle control and microtubule stabilization.
- MCC
See the MCC page for more detailed information
MCC is a gene whose product is involved in cell cycle arrest in the event of DNA damage and apparently also affects the Wnt signaling cascade. In colorectal carcinomas, its expression is often suppressed through hypermethylation of the promoter, direct mutations are not usual
- TP53
See the p53 page for more detailed information
TP53 is a tumor-suppressor gene whose product, p53, is a transcription factor playing a central role in the regulation of cell cycle progression dependent on the detection of DNA damage. Mutation of the TP53 gene is a relatively characteristic event in the late tumorigenesis of colorectal cancer.
- K-ras
See the K-ras page for more detailed information
K-ras is an oncogene whose product is a membrane protein with GTPase activity involved in signaling in a number of signaling cascades. The mutation leads to permanent activation, which subsequently leads to an increase in the transcriptional activity of a number of genes, especially BCL-2, H2AFZ, RAP1B, TBX19, E2F4 and MMP1. Numerous pathways regulating cell growth, proliferation, apoptosis, organization of the cytoskeleton and cell motility are thus affected by the increased activity of K-ras. K-ras mutation is thought to play an essential role in the transition from adenoma to carcinoma.
- Loss of 5q
Loss of 5q occurs in 20–50% of sporadic colorectal cancers. In particular, the APC and MCC genes are found in this region.
- Loss of 8p
Loss of 8p occurs in approximately 50% of colorectal cancers. Evidence of loss is more common in advanced stages, this aberration is less common in earlier stages. Loss in the 8p region increases the metastatic potential of colorectal cancer. Candidate genes are located in particular in the regions 8p21 and 8p22.
- Loss of17p
Losses in the 17p region occur in 75% of colorectal cancers, but not in adenomas. This region contains the p53 gene.
- Loss of 18q
The long arm of chromosome 18 contains a number of tumor-suppressor genes as well as genes involved in the control of cell adhesion and migration.
Pathway of microsatellite instability[edit | edit source]
Microsatellites are short repetitive sequences found throughout the genome. Their instability is a "macroscopic" manifestation of the malfunction of the "mismatch repair" system. The instability of microsatellites is manifested at the level of transcription by a shift of the reading frame. Carcinogenesis associated with microsatellite instability is associated with mutation of a number of "mismatch repair system" genes: MSH2, MLH1, MSH6, PMS2, MSH3, PMS1 and Exol; germline mutations of some of these genes are the cause of Lynch syndrome.
Five loci have been recommended since 1997 to analyze microsatellite instability: the mononucleotide repeat sequences BAT25 and BAT26 and the dinucleotide repeat sequences D5S346, D2S123, and D17S250. According to the evidence of instability, three phenotypes of microsatellite instability are distinguished:
- MSI-high (MSI-H) – instability in at least two of these loci,
- MSI-low (MSI-L) – instability in just one locus,
- MSS – all five tested loci are stable.
Alternatively, the loci BAT25, BAT26, NR21, NR24 and NR27 are used, the evaluation of which seems to have a better match with mismatch reapir gene disorders.
Another possibility of molecular evaluation is the analysis of the instability of selected tetranucleotide repetitive sequences. The phenotype with increased instability is called EMAST, corresponding to suppression of MSH3 gene expression.
MSI-H tumors are often diploid, loss of heterozygosity occurs less often, and p53 and K-ras mutations are less common. In sporadic MSI-H tumors, the V600E mutation of the BRAF gene is common. In tumors with MSI, an inactivating mutation in the TGFβRII receptor gene (TGFBR2 gene) is very common; this inactivation leads to the elimination of antiproliferative signaling by the cytokine TGF-β. [12] Mutations inactivating the antiproliferative cascade starting with the TGFβRII receptor are considered to be an essential step from adenoma to high-grade dysplasia or to cancer [13]
CpG island hypermethylation pathway[edit | edit source]
A characteristic feature of this pathway is epigenetic changes, namely hypermethylation of cytosine in CpG sequences. Pathogenetic is hypermethylation in the promoter regions of tumor-suppressor genes, which induces attenuation of their expression. Hypermethylation usually occurs in the promoter sequences of the APC, MCC, MLH1 and MGMT genes. It should be noted that it is not yet clear what is the precipitating cause of aberrant hypermethylation. [12] [13][12][13]
To analyze hypermethylation, the evaluation of methylation of CpG sequences in five marker genes is used: CACNA1G, IFG2, NEUROG1, RUNX3, SOCS1. Two levels of CpG island methylation are distinguished:
- CIMP-high (CIMP-H) – proof of methylation in at least three markers,
- CIMP-low (CIMP-L) – proof of methylation in less than three markers.
CIMP-H phenotype is often associated with BRAF mutation. [12][12]
Influence of growth factors[edit | edit source]
Growth factors from the environment are also involved to a certain extent in the formation and growth of an adenoma and later a carcinoma.
- Prostaglandins
Prostaglandin E2 (PGE2) is very strongly associated with colorectal cancer . Cyclooxygenase COX-2 participates in its synthesis , increased COX-2 activity has been demonstrated in roughly two-thirds of tumors. PGE2 is degraded by 15-prostaglandin dehydrogenase, roughly 80% of tumors have a defect in this enzyme. [13]
- EGF
More detailed information can be found on the EGF page
EGF (Epidermal Growth Factor) is a cytokine that is used in pro-growth signaling. EGFR blockade is one of the possible therapeutic modalities for advanced colorectal cancer, the blockade is ineffective in tumors with activating mutations in the EGFR pathway, especially mutations in KRAS, BRAF and the p110 subunit of phosphatidylinositol kinase (PI3K). [13]
- VEGF
More detailed information can be found on the VEGF page
VEGF (Vascular Endothelial Growth Factor) is a cytokine that stimulates vascularization during growth and neovascularization of healing tissues. It is also used in vascularization and tumor growth, its blockade is one of the possible therapeutic modalities of advanced colorectal cancer. [13]
Macroscopic Appearance[edit | edit source]
More than half of cancers occur in the rectosigmoid, right-sided tumors occur more often in elderly patients and in patients with diverticulosis. Multicentric incidence is uncommon, with a reported incidence of between three and six percent.
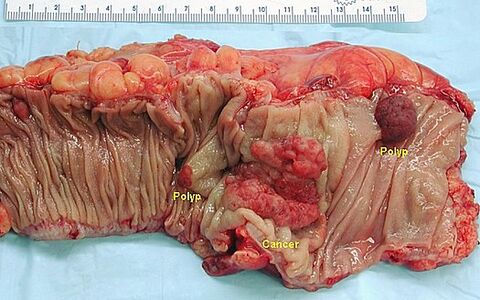
Macroscopically, colorectal cancer can present as a polypous (exophytic) or excavated (exulcerated) lesion. Polyposis carcinoma rises above the level of the mucous membrane, its edges are relatively steep and it is macroscopically well defined. An excavated carcinoma has elevated edges, and is indented and exulsed in the middle. A variant of excavated carcinoma is flat (infiltrating) carcinoma. In the right colon, the tumor can surround the wall (carcinoma annulare) and lead to stenosis.
- Macroscopic appearance of colorectal cancer
Metastatically, colorectal cancer spreads primarily via the lymphatic route to regional lymph nodes. Due to the arrangement of lymphatic vessels in the mesentery, lymphogenic metastasis can often be detected even in nodes apparently draining areas of the intestine distant from the tumor. Hematogenous metastases usually occur in advanced disease. The liver is primarily affected, other locations are less common. If the tumor grows into the peritoneum, porogenic metastases beyond the peritoneum are also possible.
Histopathology[edit | edit source]
Histologically, colorectal cancer is adenocarcinoma in more than 90% of cases, other histological types are:
- neuroendocrine tumor,
- squamous cell carcinoma,
- adenosquamous carcinoma,
- spindle cell carcinoma,
- undifferentiated carcinoma.
The basis for grading adenocarcinomas is the evaluation of the glandular formation:
- A well-differentiated adenocarcinoma is characterized by glandular formations accounting for more than 95% of the tumor. This grade represents approximately 10% of all colorectal adenocarcinomas.
- Moderately well-differentiated adenocarcinoma is characterized by glandular formations constituting 50–95% of the tumor. This grade is the most common, accounting for roughly 70% of all colorectal adenocarcinomas.
- A poorly differentiated adenocarcinoma is characterized by glandular formations accounting for less than 50% of the tumor. This grade represents approximately 20% of all colorectal adenocarcinomas.
Three-level grading is burdened with a relatively large share of subjective assessment. Therefore, some authors recommend a grading with only two grades, which has less variability of evaluation between different pathologists and which should also have a better predictive value of the prognosis:
- Low grade adenocarcinoma means that glandular formations make up at least 50% of the tumor.
- High grade adenocarcinoma means that glandular formations make up less than 50% of the tumor.
This grading can only be used for conventional adenocarcinoma, in the case of its histological changes, even a high-grade appearance can be associated with a more favorable prognostic behavior.[14]
Identifying signs of invasion is key to assessing biological behavior. If the muscularis mucosae is captured in the preparation, which may not be the rule for endoscopically collected samples, it is necessary to assess whether it is violated by a tumor. Invasive carcinoma grows through the muscularis mucosae into the submucosa, where it may have a close relationship with the submucosal vessels.
Another important sign of invasive behavior is desmoplasia or desmoplastic reaction, i.e. a type of tissue proliferation induced by invasive tumor growth. Desmoplastic reaction is characterized by the proliferation of spindle cells that surround the tumor gland.
A unique and relatively frequently present feature of colorectal cancer is necrotic detritus in the lumen of tumor glands, sometimes referred to as dirty necrosis. These can also appear in metastases, so they represent an important clue in determining the origin of tumors of unclear origin.
In the case of colorectal cancer, only cancer with submucosa invasion should be designated as invasive cancer, i.e. stage pT1. Tumors growing into the muscularis mucosae without further ingrowth have little potential to establish distant or lymph node metastases. The biological reason for this behavior of a tumor growing at the highest point in the muscularis mucosae is not exactly known, it is assumed that a relatively poor network of lymphatic vessels plays its role. According to the AJCC Cancer Staging Manual (7th edition), a tumor growing highest into the muscularis mucosae should be referred to as carcinoma in situ (pTis), some authors even recommend the designation of high grade dysplasia so that the terminology does not give the impression of the need for further surgery.[14]
- Microscopic appearance of colorectal adenocarcinoma
Colorectal adenocarcinoma can have several histological variants:
- mucinous adenocarcinoma,
- adenocarcinoma with signet ring-shaped cells,
- medullary adenocarcinoma,
- micropapillary adenocarcinoma,
- serous adenocarcinoma,
- cribriform comedone adenocarcinoma,
- adenosquamous adenocarcinoma,
- spindle cell adenocarcinoma,
- undifferentiated adenocarcinoma.
- Mucinous Adenocarcinoma
A defining feature of mucinous adenocarcinoma is that at least 50% of the tumor volume is composed of extracellular mucin. Carcinomas that contain less mucin but still more than 10% of the volume are referred to as adenocarcinoma with mucinous differentiation or adenocarcinoma with mucinous features.
Histologically, mucinous adenocarcinoma usually forms relatively large glandular masses with pools of extracellular mucin. Different numbers of individual tumor cells may be present, and signet ring-shaped cells may also be detected.
The biological behavior of mucinous adenocarcinoma is uncertain. As for the tumor arising on the basis of Lynch syndrome, it has an MSI-H microsatellite phenotype and its behavior can be assessed as low-grade. On the other hand, if mucinous adenocarcinoma is associated with the MSS microsatellite phenotype, its biological behavior is distinctly more aggressive.[14]
- Signet Ring Cell Adenocarcinoma
A defining feature of signet ring cell adenocarcinoma is that at least 50% of the tumor cells have signet ring cell features. A characteristic feature of signet ring-shaped cells is a bulky central vacuole filled with mucin and pushing the nucleus to the edge of the cell. Infiltrative growth or entrapment of free cells in pools of extracellular mucin is common. Signet ring cell adenocarcinoma usually behaves as a high-grade tumor, but low-grade behavior can be expected if the MSI-H phenotype is demonstrated.[14]
- Medullary Adenocarcinoma
Medullary adenocarcinoma is characterized by bands of epithelioid cells with bulky vesicular nuclei, distinct nucleoli, and abundant cytoplasm. Infiltration of the tumor by abundant lymphocytes is relatively common. Cell differentiation is usually poor, cells may be undifferentiated. The MSI-H microsatellite phenotype is relatively common. Despite the usually poor differentiation, the prognosis is relatively good.[14]
Clinical Management[edit | edit source]
Clinical Picture[edit | edit source]
Colorectal cancer, especially right-sided cancer, is often clinically silent in its early stages and can only be detected during screening. Typical manifestations are bleeding into the colon and changes in bowel movements (e.g. alternating diarrhea and constipation). Chronic blood loss can lead to anemia. Another relatively common symptom is non-specific and rather vague abdominal pain. On the left side and less often in the colon ascendens and in the cecum, one of the first manifestations of the tumor can be obstruction of the large intestine.
Screening[edit | edit source]
For more detailed information, see Colon Cancer Screening
Včasný záchyt kolorektálního karcinomu představuje faktor, který výrazně zlepšuje prognózu nemocného. Cílem screeningu je nejen odhalit již vzniklou malignitu, ale i premaligní změny. Optimální načasování jednotlivých screeningových metod je otázkou stále probíhajícího výzkumu.[5]
Occult Bleeding[edit | edit source]
For more detailed information, Immunochemical test of blood in stool
Fecal Occult Blood (FOB) is a condition in which there is an admixture of blood in the stool in excess of physiological blood loss into the stool. The test is relatively cheap, not burdensome, but less sensitive. Nevertheless, its regular implementation at an interval of two years has the potential to reduce mortality associated with colorectal cancer by up to 16%.[5]
Sigmoidoscopy[edit | edit source]
Examination of the rectosigmoid with a flexible 60 cm sigmoidoscopy allows to detect about 60% of colorectal cancers. The advantage over colonoscopy is the easier preparation of the patient, an enema is sufficient for the procedure , as well as a lower risk of complications.[5]
Colonoscopy[edit | edit source]
More detailed information can be found on the Colonoscopy page
Colonoscopy is the diagnostic gold standard because, in addition to overlooking the mucosa of practically the entire colon, it also allows for the collection of samples from suspicious lesions and the excision of premalignant lesions.
Virtual colonoscopy[edit | edit source]
More detailed information can be found on the Virtual Colonoscopy page
Virtual colonoscopy (CT colonography) may be suitable for assessing the exact location of the tumor, especially in cases where colonoscopy cannot be performed. The disadvantage is the patient's radiation exposure.
Capsule colonoscopy[edit | edit source]
More detailed information can be found on the Capsule Endoscopy page
Capsule colonoscopy is a screening method that involves swallowing a capsule capable of taking images of the digestive tract. The main advantage compared to classic colonoscopy is higher patient comfort (and therefore higher compliance) and lower risk of complications, the disadvantage is the impossibility of taking a sample for histological examination and the time-consuming evaluation of the record. If a suspicious lesion is detected, a classic colonoscopy should be performed.[15]
Molecular Biological Tests[edit | edit source]
In the research phase, screening tests are based on DNA analysis in blood or stool.[5]
Diagnostics[edit | edit source]
The diagnosis is established on the basis of sigmoidoscopy or colonoscopic examination with sample collection for histological examination. A newly diagnosed tumor should be followed by additional investigations (if not already performed):[5]
- physical exam,
- complete colonoscopy to rule out metachronous carcinoma in another part of the colon,
- CT scan of the lungs, abdomen and pelvis to detect possible metastatic involvement.
In the case of rectal cancer, MR examination is more useful, especially with regard to assessing the spread of the tumor into the mesorectum and thus assessing the appropriate extent of resection. In the case of early tumors, endoscopic ultrasound examination can be used to assess invasion. Both of these methods have their advantages and limitations. An ultrasound examination is advantageous for examining the liver, and the use of ultrasound contrast agents appears promising. Carcinomatosis of the peritoneum remains a diagnostic problem.[5]
ICD[edit | edit source]
- C18: Malignant neoplasm of the colon.
- C18.0: Cecum and ileocecal valve.
- C18.1: Appendix.
- C18.2: Colon ascending.
- C18.3: Flexura hepatica
- C18.4: Colon transversum.
- C18.5: Flexura lienalis.
- C18.6: Colon descendens.
- C18.7: Colon sigmoideum.
- C18.8: The lesion extends beyond the intestine.
- C18.9: Not specified.
- C19: Malignant neoplasm of the rectosigmoid junction.
- C20: Malignant neoplasm of the anus.
- C21: Malignant neoplasm of anus and anal canal.
- C21.0: Anus, unspecified.
- C21.1: Anal canal.
- C21.2: Cloacogenic zone.
- C21.8: The lesion extends beyond the rectum, anus, and anal canal.
Staging[edit | edit source]
For an adequate determination of lymph node involvement, at least 12 lymph nodes should be examined.[5] Technical conditions sometimes do not allow this, and therefore it is only considered a sign of poor pathology work if the average number of examined nodes in a larger number of resections falls significantly below 12.
- Clinical stages
- stage 0: TisN0M0
- stage I: T1N0M0 nebo T2N0M0
- stage IIA: T3N0M0
- stage IIB: T4N0M0
- stage IIIA: T1N1M0 nebo T2N1M0
- stage IIIB: T1N2M0, T2N2M0 nebo T3N1M0
- stage IIIC: T3N2M0, T4N1M0,T4N2M0
- stage IVA: any T and N, distant metastases in one anatomical location
- stage IVB: any T and N, distant metastases in more than one anatomical location
- Dukes classification
- stage A: tumor confined to the intestinal wall
- stage B: the tumor invades or penetrates the serosa
- stage C: involvement of the lymph nodes
- stage C1: positive pericolic lymph nodes
- stage C2: positive perivascular nodes
- stage D: distant metastases
Typing & Grading[edit | edit source]
Typing and grading are governed by the WHO classification of colorectal tumors (vč. ICD-O codes, abbreviated):[16]
- Carcinomas:
- adenocarcinoma 8140/3
- mucinous adenocarcinoma 8480/3
- Signet ring cell carcinoma 8490/3
- Small cell carcinoma 8041/3
- Squamous cell carcinoma 8070/3
- Adenosquamous carcinoma 8560/3
- Medullary carcinoma 8510/3
- undifferentiated carcinoma 8020/3
- Carcinoid 8240/3
- EC cells, serotonin-producing neoplasia 8241/3
- Mixed carcinoid-adenocarcinoma 8244/3
- Non-epithelial tumors
- gastrointestinální stromální tumor 8936/1
- gastrointestinal stromal tumor 8936/1
- leiomyosarcoma 8890/3
- angiosarcoma 9120/3
- Kapisi sarcoma 9140/3
- malignant melanoma 8720/3
- lymphomas
Therapy[edit | edit source]
Surgical therapy[edit | edit source]
Surgical therapy represents the basic therapeutic modality. If it is chosen, it is advisable to perform a sufficiently radical performance incl. lymphadenectomy. The resection margin should be at least 5 cm away from the tumor if possible; some authors consider a distance of 2 cm from the distal edge of the rectal resection to be sufficient. The extent of resected fat tissue should be such that at least 12 lymph nodes can be examined. In the case of T4, only a sufficiently extensive "en bloc" resection can be considered a resection with intact resection margins. Mechanical bruising of the tumor during resection can cause its spread, which is why the surgical technique is adapted to prevent such spread - the no-touch concept. If the tumor is traumatized during the procedure, adjuvant chemotherapy is appropriate.[5][4]
For invasive rectal cancer, total mesorectal excision (TME) with adequate circular and distal resection margins and excision of lower mesenteric lymph nodes is the method of choice. Sphincter-sparing procedures are intended for patients in a lower stage of the disease, in very early stages (T1Sm1) local excision can also be considered.[5]
For colon tumors, laparoscopic colectomy is the method of choice . From an oncological point of view, the results of a laparoscopic procedure can be considered the same as laparotomic procedures, the laparoscopic procedure is more advantageous for the patient.[5]
Resection of liver and lung metastases, if they are formed, significantly increases the survival of patients. In addition to surgical resection, e.g. local embolization or radiofrequency ablation is used; these methods increase the number of patients who can be treated for metastases. Metastases can also be targeted with neoadjuvant chemotherapy.[5]
For unresectable tumors or for tumors of patients inoperable due to comorbidities, palliative procedures, especially bypass operations and diversion stoma, are considered. These procedures can improve the patient's quality of life and, in conjunction with appropriate pharmacotherapy, survival.[4]
Radiotherapy[edit | edit source]
Neoadjuvant radiotherapy makes it possible to perform surgery at all in patients at a higher stage, but by itself it has no effect on overall survival.[4]
Pharmacotherapy[edit | edit source]
Neoadjuvant chemotherapy is usually performed with 5-fluorouracil, sometimes combined with radiotherapy. .[17]
Adjuvant chemotherapy improves survival in II. stage by several percent, in III. stage already by 15-20%. For that reason, in III. stage of adjuvant chemotherapy fully indicated. In addition to 5-fluorouracil, oxaliplatin is also used. Biological therapy is not indicated in adjuvant therapy.[17]
Therapy IV. stage is a complex issue, the procedure is chosen according to the patient's condition and the extent of metastatic involvement. In addition to cytostatics ( 5-fluorouracil , oxaliplatin , irinotecan , fluoropyrimidine ), biological therapy can also be used if its effectiveness can be assumed from the results of the molecular examination. Currently available are:[17]
- the EGFR receptor inhibitors cetuximab and panitumumab,
- anti-VEFG antibody bevacizumab,
- aflibercept – circulating VEGF binding protein,
- inhibitor of protein kinases of a number of signaling cascades regorafenib.
Prognosis[edit | edit source]
The prognosis of adenocarcinoma is similar to rectal and colon cancer, and depends mainly on the clinical stage. Five-year survival is as follows: [5]
- stage I: 97.1 %
- stage IIA: 87.5 %
- stage IIB: 71.5 %
- stage IIIA: 87.7 %
- stage IIIB: 75.0 % (if N1), 68.7 % (if N2)
- stage IIIC: 47.3 % (T3, N2), 50.5 % (T4, N1), 27.1 % (T4N2)
In the fourth stage, the prognosis depends on which organs are affected metastatically, what is the extent of metastatic involvement and what is the general condition of the patient. Overall, however, the prognosis is not very favorable, and at this stage, I usually consider colorectal cancer to be incurable in the sense of curative therapeutic intent when choosing therapeutic modalities.[13]
Links[edit | edit source]
Reference[edit | edit source]
3. STROUHAL, E. and A. NÉMEČKOVA. Did ancient people also suffer from cancer? : History and paleopathology of tumors, especially malignant ones. 1st edition. Prague: Karolinum, 2008. ISBN 9788024614816 .
4. MULCAHY, HE, J. HYLAND and DP O'DONOGHUE. From dinosaurs to DNA: a history of colorectal cancer. Int J Colorectal Dis. 2003, vol. 18, no. 3, pp. 210-5, ISSN 0179-1958.
5. CRAWFORD, K., et al. European Sexualities, 1400-1800. 1st edition. Cambridge University Press, 2007. 246 pp. New Approaches to European History; St. 38. Chapter 5 John Burrows and the Vegetable Wars. pp. 85–102. ISBN 9780521839587.
6. KALA, Z., P. KYSELA and L. OSTRÍŽKOVÁ, et al. Surgical and minimally invasive treatment of colorectal cancer. Oncology [online] . 2011, vol. 5, no. 5, pp. 270-272, also available from < http://www.onkologiecs.cz/pdfs/xon/2011/05/07.pdf >. ISSN 1803-5345.
7. CUNNINGHAM, D., W. ATKIN and HJ LENZ, et al. Colorectal cancer. Lancet. 2010, vol. 375, no. 9719, pp. 1030-47, ISSN 1474-547X.
8. LAZARUS-BARLOW, WS and JH LEEMING. The natural duration of cancer. Br Med J [online] . 1924, vol. 2, no. 3320, pp. 266-7, also available from < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2304825/?tool=pubmed >. ISSN 0007-1447.
9. WYARD, S.. The natural duration of cancer. Br Med J [online] . 1925, vol. 1, no. 3344, pp. 206-7, also available from < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2196841/?tool=pubmed >. ISSN 0007-1447.
10. G. VEPŘEKOVÁ and O. MÁLEK, et al. Epidemiology, etiology, screening and diagnosis of colorectal cancer, including diagnostic and therapeutic interventions on the colon. Oncology [online] . 2011, vol. 5, no. 5, pp. 261-265, also available from < http://www.onkologiecs.cz/pdfs/xon/2011/05/05.pdf >. ISSN 1803-5345.
11. GLOBOSCAN 2012: Fact sheet population
12. Epidemiology of malignant tumors in the Czech Republic
13. JASS, JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology.. 2007, vol. 50, no. 1, pp. 113-30, ISSN 0309-0167.
14. AL-SOHAILY, S., A. BIANKIN, and R. LEONG, et al. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol.. 2012, vol. 27, no. 9, pp. 1423-31, ISSN 1440-1746.
15. MARKOWITZ, SD and MM BERTAGNOLLI. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med [online] . 2009, vol. 361, no. 25, pp. 2449-60, also available from < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843693/?tool=pubmed >. ISSN 1533-4406.
16. FLEMING, M., S. RAVULA and SF TATISHCHEV, et al. Colorectal carcinoma: Pathological aspects. J Gastrointest Oncol [online] . 2012, vol. 3, no. 3, pp. 153-73, also available from < http://jgo.amegroups.com/article/view/410/html >. ISSN 2219-679X.
17. M. VOŠKA and G. VEPŘEKOVÁ, et al. Colon capsule in the context of colorectal cancer screening. Oncology [online]. 2012, vol. 6, no. 3, pp. 165-168, also available from < http://www.onkologiecs.cz/pdfs/xon/2012/03/08.pdf >. ISSN 1803-5345.
18. HAMILTON, Stanley R. and Lauri A. AALTONEN. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System, [online]. 1st edition. Lyon : IARC Press, 2000. Also available from < http://publications.iarc.fr >. ISBN 92-832-2410-8.
19. A J. HALÁMKOVÁ. Systemic antitumor treatment of colorectal cancer. Oncology [online]. 2013, vol. 7, no. 4, pp. 188-189, also available from < http://www.onkologiecs.cz/pdfs/xon/2013/04/07.pdf >. ISSN 1803-5345. >
Virtual Preparations[edit | edit source]
Related articles[edit | edit source]
- Colorectal Cancer
- Treatment of liver metastases in colorectal cancer
- Colon polyps
- Vienna Classification of Gastrointestinal Neoplasia (2002)
Literature[edit | edit source]
External Links[edit | edit source]
- Dušek, L., Zavoral, M., Májek, O., Suchánek, Š., Mužík, J., Pavlík, T., Šnajdrová, L., Gregor, J. Kolorektum.cz – Program kolorektálního screeningu v České republice [online]. Masarykova univerzita, Brno, 2014. Dostupné z WWW: [1]. ISSN 1804-0888.
Kategorie:Gastroenterologie Kategorie:Chirurgie Kategorie:Patologie Kategorie:Onkologie