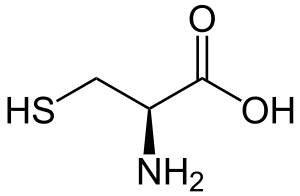
Cysteine
Cysteine is a non-essential amino acid that, like methionine , has a sulfur atom in its molecule.
Metabolism cysteine[edit | edit source]
Cysteine synthesis takes place in the human organism from homocysteine and serine .
The degradation of cysteine begins with the oxidation of the -SH group to -SO2- by the enzyme cysteine dioxygenase. In the resulting L-cysteine sulfinate , the -NH2 group is replaced by a keto group with the help of transaminase , and β-sulfinylpyruvate is formed . In the final reaction, it is split by desulfinase into pyruvate and sulfite (SO3-), or final sulfate (SO4-). Cysteine is an important source of taurine . L-cysteine sulfinate is decarboxylated to hypotaurine and subsequent oxidation of the -SO2- - group to -SO3- produces taurine.
Alternative non-oxidative degradation of cysteine produces pyruvate and sulfane (H2S).
Importance[edit | edit source]
- In peptides, cysteine is essential for the formation of disulfide bridges.
- It is a substrate for glutathione.
- It is a substrate for taurine. The latter is conjugated with bile acids or other substances that increase their solubility in water.
- Decarboxylation of cysteine produces cysteamine , which is part of coenzyme A.
- It has a high proportion of keratin protein (hair, nails).
Links[edit | edit source]
Related articles[edit | edit source]
References[edit | edit source]
- MATOUŠ, Bohuslav, et al. Fundamentals of medical chemistry and biochemistry. 2010 edition. Prague: Galen, 2010. 0 pp. ISBN 978-80-7262-702-8 .