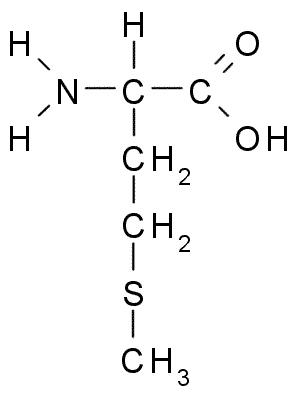
Methionine
Methionine is an essential, nonpolar proteinogenic amino acid containing a sulfur atom. It can be converted to cysteine.
Metabolism[edit | edit source]
As an essential amino acid, methionine cannot be synthesized and must be obtained from the diet.
Convert to SAM[edit | edit source]
S-adenosylmethionine, or active methionine, is a nucleoside capable of methylating other substances and is therefore essential in many metabolic pathways (choline, creatine, adrenaline, ...). This property is due to the presence of a positively charged sulfur atom. The synthesis of SAM from methionine requires ATP and is catalyzed by the appropriate transferase.
Conversion to Cysteine [edit | edit source]
The starting substance of conversion to cysteine is S-adenosylmethionine. This is converted into S-adenosylhomocysteine by splitting off the methyl, which then changes into homocysteine. After its fusion with serine, cystathion is formed. It disintegrates immediately, but the sulfur atom remains on the serine skeleton. Homocysteine is left to homoserine, which is converted to succinyl-CoA via propionyl-CoA.
Task in translation[edit | edit source]
As methionine is encoded by the initiation codon AUG, it forms the cap at the N-terminus of each newly synthesized peptide. However, this is usually removed as part of post-translational modifications.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- KIDNEY, Miroslav – STOKLASOVÁ, Alena – CERMAN, Jaroslav. Biochemistry for medical students. 2. edition. Prague : Karolinum, 2009. ISBN 978-80-246-1414-4.
- MURRAY, Robert Kincaid – BOTHAM, Kathleen M, et al. Harper's Illustrated Biochemistry. 5. edition. Prague : Galen, 2012. ISBN 978-80-7262-907-7.