Plasma proteins
The proteins in blood serum or plasma are many and are produced by various cells. The biosynthesis of the vast majority of plasma proteins takes place in the liver. A smaller part is synthesized in other cells: e.g. lymphocyte (immunoglobulins) and enterocytes (e.g. apoprotein B-48). Protein degradation takes place inhepatocytes and the mononuclear phagocytic system, where proteins are degraded predominantly after complex formation (e.g. antigen-antibody complex and hemoglobin-haptoglobin complex). Intracellularly, peptide bonds of proteins are hydrolyzed by proteases and peptidases to form amino acids. Another way serum proteins are removed is via excretion, which is facilitated by the kidneys and the gastrointestinal tract.
The total serum concentration of proteins is 65-85 g/L. Because plasma proteins are osmotically active, their physiological concentration contributes to a colloid osmotic pressure (oncotic pressure) of 3.33 to 3.52 kPa (25 to 26.4 torr). The concentration of proteins in plasma is slightly higher than in serum because plasma contains coagulation factors.[1]
Functions of plasma proteins
Plasma proteins are necessary for a variety of blood/plasma functions:
- Maintenance of oncotic pressure
- Transport of lipophilic compounds, such as some hormones (thyroid hormone bound to transthyretin), vitamins, lipids (bound to albumin), bilirubin bound to albumin) and drugs.
- Nutrition function
- Maintaining acid-base balance
- Hemocoagulation a fibrinolysis
- Humoral immunity
- Specific immunity (immunoglobulins)
- Non-specific immunity (complement, acute-phase proteins)
Overview of plasma proteins
|
Albumin
Albumin is the most common serum protein, it accounts for approximately 55 to 65% of total serum proteins (average blood concentration is 40 g/L[2]). Je syntetizován v játrech a jeho tvorba závisí na příjmu aminokyselin.
- Albumin is crutial for maintenance of the oncotic pressure of the plasma. Decreased albumin concentrations (hypoalbuminemia) below 20 g/L usually lead to oedemas.
- It acts as a carrier. It enables the transport of bilirubin, heme, steroid compounds, tyroxine, fatty acids, bile acids, metals, drugs, among others.
- Albumin is a protein reserve of the body and serves as a source of amino acids, especially essential amino acids for various tissues. During malnutrition, its concentration decreases, however, serum albumin levels are not a good indicator of early protein malnutrition because albumin has a long plasma half-life and large body stock. For this reason, albumin is a long-term marker of nutrition.
Synthesis of albumin
The synthesis of albumin has multiple steps. Preproalbumin, a precursor of albumin is synthesized by hepatocytes, but does not exit the cell cytoplasm. Subsequently, preproalbumin enters endoplasmatic reticulum, where it is transformed into proalbumin, the most abundant intracellular form of albumin. Then, proalbumin enters Golgi complex, where it is transformed into albumin and excreted our of the cell.[3]
Acute-phase protein
Acute-phase protein are proteins that are increased during acute inflammatory reaction of the organism, trauma, surgiceries, infarct of myocard, tumours, birth, increased stress of physical exercise. All abovementioned situations can induce the increase of acute-phase proteins, but the molecular response will vary based on the underlying cause (i.e. bacterial inflammatory reaction will increase different proteins than e.g. trauma).
Such mediators serve to ensure the overall response of the organism, mutual communication and regulation of ongoing events. Additionally, they also produces the "general symptoms" of inflammatory process (fever, muscle and joint pain). These substances are of clinical importance, the main synthesis arises as a result of known pathology, or when its concentration corresponds to the extent of tissue damage. Therefore, these substances may be used as markers for confirming/determining the origin of organism damage (whose symptoms may be relatively non-specific), determining the extent of the damage, and monitoring the course of the therapy.
Positive markers of acute phase response
This group encompases various proteins, whose blood (plasma) levels increase upon the beginning of the pathological process. They can be divided into following groups based on their effect on the organism (or their purpose in organism):
Immune complexes
- The purpose of some proteins of acute phase is to neutralize the xenobiotics (or microorganism) that caused the inflammation. Examples may be:
- C-reactive protein (CRP),
- complement, (all complement cascade proteins are increased, C3 and C4 have the higher physiological plasma concentrations, therefore, their increase if the most pronounced),
- tumor necrosis factor α (TNF-α), interleukin 1 (IL-1) and interleukin 6 (IL-6).
Proteins that prevent the colateral damage of the inflammatory response
- During inflammation, immune cells (such as phagocytes) release cytotoxic compounds that may damage not only the pathogen, but also the healthy tissues, which would cause undesirable side effects. To avoid this colateral damage, during inflammatory response, organism produces proteins that excessive inactivate proteolytic enzymes and reactive oxygen species (ROS) mitigating the damage to its own tissues. Such compounds include
- Protease ingibitors
- Compounds that decrease the synthesis or concentration of reactive oxygen species (ROS) - not only ROS scavengers, but also proteins that bind metal (mostly iron and copper) whose presence may worsen the inflammatory process. As a resuls, chelation of iron and copper may decrease the synthesis of reactive oxygen species (via Fenton reaction). Such compounds include
- Compounds, whose purpose is the transport of waste products away from the inflammation. Such compounds include
-
- hemoglobin
- hemopexin
- serum amyloid A (SAA).
- Coagulation factors and proteins inducing the tissue regeneration. Such compounds include
The purpose of some positive reactants, such as procalcitonin (PCT), remains unknown. Despite the unknown purpose, its increase of plasma concentration may be of a key importance when determining the nature of acute phase response. Therefore, even some compounds with unknown physiological function may be clinically useful.
The rate of increase of acute-phase proteins
The rate of increase of acute-phase proteins varies considerably. Therefore, for clinical purposes, we can divide acute-phase proteins into three groups: early, intermediate, and late based on the rate of increase of their plasma concentrations.
Early positive acute-phase proteins
Early positive acute-phase proteins jsou bílkoviny s velmi krátkým biologickým poločasem. Změny jejich plazmatické koncentrace jsou patrné již za 6–10 hodin po začátku onemocnění. Vzestup vrcholí obvykle v průběhu druhého a třetího dne. Hlavními představiteli jsou především C-reaktivní protein (CRP) a sérový amyloid A (SAA). Nověji se v klinické praxi používá prokalcitonin (PCT).
- C-reactive protein
C-reactive protein (CRP) is one of the most important acute-phase proteins in diagnostics. This protein primarily functions to activate opsonine - it forms insoluble complexes (precitipates) with C-polysaccharide of pneumococus (thus earning the name C-reactive protein).[4]
Physiologically, the plasma concentration of C-reactive protein should not exceed 8 mg/L.[5] Acute bacterial infections (and rarely mycotic inflammatons) cause a quick and sharp increase of CRP (usually above 60 mg/L, mostly up to 200 mg/L, the higher concentrations reveal higher extent of infection). On the other hand, viral infection usually lead to only minor increase of C-reactive protein increase in plasma (usually bellow 40 mg/L, sometimes the CRP values remain unchanged).[6] Plasma concentration of C-reactive protein increases as early as 4 hours after the beginning of acute phase. Moreover, withing first two days of acute phase, its concentration can increase to more than 1000-fold its physiological concentration. Peak concentration is reached between 24 and 48 hours. The determination of plasma concentration of CRP, therefore, is helpful in early decision with antibiotic therapy.[4] Additionally, the biological half-life of C-reactive protein is approximatelly 24 hours, therefore, CRP concentrations in plasma closely reflect the course of acute phase.[7] During a sucessful antibiotic therapy, CRP plasma levels will quickly decrease, whereas during a non-sucesfull antibiotic therapy, the CRP levels will remaing high or keep growing.
Plasma concentrations of CRP can be used to identify a beginning post-surgery infections. Although CRP may be elevated after the surgery even without any infection, the third day after the infection, the CRP values should return to normalcy. If the return of CRP to its physiological values are slow (or absent), this may be an indication of an ongoing infection.
A mild increase of CRP may be observed in myocardial infarction. Generally speaking, mildly elevated CRP values (usually up to 10 mg/L) may be a marker of increased cardiovascular risk.[8] CRP values may be increased in autoimmune diseases and may be used in their long-term monitoring.[9]
Disadvantage of CRP is its relatively low specifity. Additionally, CRP does not reflext the magnitude of the magnitude as well as procalcitonin does. These two markers are, to an extent, complementary.
Recently, procalcitonin (PCT) has reached a major interest in clinical practice. This 116-aminoacid long protein (molar mass 13,000 g/mol) is synthesized by the C cells of the thyroid gland as a precursor of calcitonin hormone. During an inflammation (generalized bacterial inflammations), this molecule can be synthesized by other cells throughout the body, especially by neuroendocrinne cells of lungs and intestine, but also in other organs [10]. As a result, during a bacterial infection, the plasma concentration of this protein increases. Procalcitonine synthesized during infection or sespis is not converted into calcitonin.[11] The exact patophysiological role of procalcitonin remains unknown, but it has been speculated that it can play a role in inflammation regulation and that it has analgetic effect. The half-life of procalcitonin is 1 day, its concentration in plasma rises as soon as 2 to 3 hours up to 20-fold of its physiological values. The increase of procalcitonine concentrations can be seen only in generalized bacterial, mycotic or protozoal infections, but not during viral infections. A minor increase of PCT in plasma can be seen after polytrauma, burn patients or after major surgeries of the abdomen.
Assessment of PCT
PCT can be assessed using highly sensitive PCT-LIA (Luminescence ImmunoAssay). This method uses two monoclonal antibodies, one of which has affinity towards C-terminal sequence of procalcitonin, and the other antibody has affinity towards the central part of the procalcitonin. Antibodies against the C-terminal sequence are immobilized on the surface of the test tube, while the antibodies agains the central part of procalcitonine are soluble and they are traced with fluorescent trace. This method requires a luminometer, and approximately 20 μL of serum or plasma.
A quick method for assessment of PCT is immunochromatography for procalcitonin (PCT-Q). This method requires 200 μL of serum or plasma, the result is available within 30 minutes. This test is recommended for quick diagnostics of acute pancreatitis.
Orientační hodnoty PCT
Normální hodnoty (ng/ml) < 0,5; chronické zánětlivé procesy < 0,5–1; bakteriální infekce komplikovaná systémovou reakcí 2–10; SIRS 5–20; těžké bakteriální infekce – sepse, MODS 10–1000. Při protrahované sepsi přetrvává zvýšená hladina PCT, zatímco hladiny některých jiných cytokinů klesají.[11]
Non-infection reasons for increased PCT
PCT can be elevated after surgeries, multiple traumas, heat-induced tissue damage or cardiogennin shock. Additionally, elevated PCT can be seen in neonates within firts 48 hours past birthu.[11]
The comparison of PCT, CRP, IL-6, and WBC shows (all of which can be used as markers of accute inflammation) PCT is both the most sensitive and selective marker to diagnose and destinguish bacterial and non-bacterial infections.[12]
Intermediate potisive markers of acute-phase
Intermediate potisive markers of acute-phase are proteins whose concentration increases 12 to 36 hours after the beginning of the acute phase and peaks at the end of the first week. This group encompasses orosomukoid, α1-antitrypsin, haptoglobin and fibrinogen.
Late positive markers of acute-phase
Late positive markers of acute-phase encompass complement proteins C3 and C4, and ceruloplasmin, among others. Their plasma concentrations increase 48 to 72 hours after the beginning of acute phase and peaks 6 to 7 days after the beginning of the acute phase. These markers are not as valuable in diagnostics as early markers of acute phase, but they can inform us that an inflammation has been present recently or that the infection has been progressing for a long time.
Negative markers of acute-phase
Acute phase negative reactants are the proteins whose levels decreases during acute exercise. The main examples are albumin, prealbumin and transferrin. Such markers are diagnostically less important than positive reactants for monitoring and evaluating the course of the load response. However, they are often used as a criterion for protein synthesis in the liver and as indicators of malnutrition.
Immunoglobulins
Antibodies (immunoglobulins) are specific blood plasma globulins with β-γ electrophoretic mobility. They are synthesized in plasma cells as a humoral component of the immune response to a particular antigen. An immunoglobulin molecule has the ability to specifically bind the appropriate antigen against which it has been generated. After binding, an immune complex is formed. In addition, immunoglobulins perform other functions including, for example, complement binding, binding to neutrophilic leukocytes and macrophages, activation Phagocytosis. Immunoglobulins are divided into 5 classes IgG, IgM, IgA, IgD and IgE. In addition, IgG classes have been divided into subclasses - IgG-1, IgG-2, IgG-3, IgG-4, whose functions differ. Also, the IgA class has 2 subclasses: IgA-1 and IgA-2. The basic structure of an immunoglobulin molecule consists of two identical heavy chains (H-chains), denoted by the individual classes γ, μ, α, δ and ε, and two light chains (L-chains) κ and λ, which are common to each class. Each immunoglobulin molecule contains either κ or λ chains. At the first infection with bacteria or protozoa, IgM antibody production occurs within 2 to 3 days, which is later replaced by IgG production with the same specificity within 5 to 7 days. Repeated infection will cause a rapid increase in IgG levels and a small increase in IgM levels. Significant changes in the amount of immunoglobulins are manifested in the electrophoretic examination as:
- hypogammaglobulinemia (reduction of the peak in the γ region);
- hypergammaglobulinemia;
- polyclonal (broad base β-γ globulin peak increase);
- monoclonal (narrow peak in the region of β-γ globulins).
Hypogammaglobulinemia
Hypogammaglobulinemia (decreased levels of immunoglobulins) results from decreased immunoglobulin synthesis or increased immunoglobulin losses (urine or intestine). It can be an isolated defect of individual components (IgA, IgM, ...), but they can also affect all components at the same time. These humoral immunity defects can be primary or secondary and are the cause of severe immunodeficiency conditions manifested by recurrent, severe infections.
Methods of assessing the protein concentrations
A basic measurement of proteins in plasma or serum is the assessment of total serum/plasma protein. However, the information value of this parameter is rather limited. A higher information value can be reached if the proteins are first separated (usually via electrophoresis) and then quantified.
Serum protein electrophoresis
Principle
Serum protein electrophoresis (SPEP) is a separation method based on a movement of charged particles within electric field. The compounds of interest need to be charged (i.e. they must either be ions, or ampholytes). Most proteins have ampholytic nature, therefore, their net charge can be positive or negative with variance of pH of the buffer during electrophoresis. Once a mixture of various charged molecules is exposed to a stationary electric field, individual ions will start moving towards either electrodes. The velocity of movement of ions depends on following factors:
- the charge of the molecule (positive ions move towards negative electrode, negative ions move towards positive electrode).
- magnitude of charge (the higher charge, the more the molecule is attracted to the electrode; if the net charge of a molecule is equal to zero, the molecule will not move at all)
- size of the compound or relative molar mass of the compound (molecules with higher molar mass will move slower that those with lower molar mass)
- voltage
Usually, a mixture of protein is separated in electrophoresis at pH 8.6 (using akaline buffer). Because izoelektric point of most serum proteins is near 5 to 6, at pH 8.6, all proteins are negatively charged, therefore, they will move towards the anode (positive electrode).
Blood serum electrophoresis usually separates proteins into 6 to 7 fractions: prealbumin (seen rarely), albumin, α1, α2, β1, β2, (sometimes poorly resolved, may be seen only as β fraction), γ fractions. With the exception of albumin and prealbumin fractions which contain only single protein each, these fractions consist of multiple proteins with similar electrophoretic mobilities.
Plasma protein concentration by electrophoretic fraction
| Fraction | Relative protein concentration (%) | Absolute protein concentration (g/L) |
|---|---|---|
| Albumin | 55 to 69 | 35 to 44 |
| α1 | 1.5 to 4 | 1 to 3 |
| α2 | 8 to 13 | 5 to 8 |
| β | 7 to 15 | 4 to 10 |
| γ | 9 to 18 | 5 to 12 |
Clinical use
Serum protein electrophoresis (SPEP) is used especially if we find a pathological result of the total protein, or if we need more detailed information about serum proteins. Especially valuable for the card:
- dysproteinemia - change in the concentration and qualitative composition of individual proteins in serum,
- paraproteinemia - characterized by the presence of monoclonal immunoglobulins.
| Electrophoresis results | Comment | Alb | α1 | α2 | β | γ | Examples of common pathologies |
|---|---|---|---|---|---|---|---|
| Acute inflammation |
|
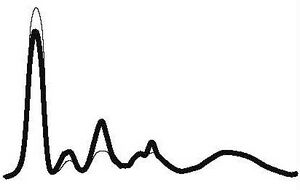
|
| ||||
| ↓ or N | ↑ | ↑ | N | ||||
| Chronic inflammation |
|
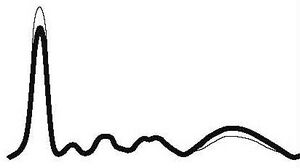
|
| ||||
| ↓ or N | N | N | N | ↑ | |||
| Chronic active inflammation |
|
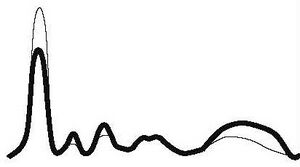
|
| ||||
| ↓ | ↑ | ↑ | N | ↑ | |||
| Hepatic pathology |
|
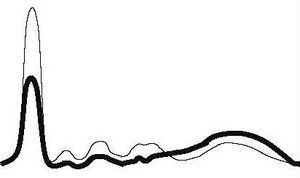
|
| ||||
| ↓ | ↓ | ↓ | ↓ | ↑ | |||
| Nephrotic pathology |
|
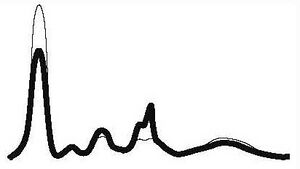
|
| ||||
| ↓ | N | ↑ | ↑ | ↓ or N | |||
| Hypogamaglobulinemia |
|

|
| ||||
| N | N | N | N | ↓ | |||
| Monoclonal gamapathy |
|

|
| ||||
| ↓ | ↓ | ↓ | ↑ | ↑ | |||
Links
Reference
- ↑ BURTIS, Carl A a Edward R ASHWOOD. Tietz textbook of clinical chemistry. 2. vydání. Philadelphia : Saunders, 1994. 2326 s. ISBN 0-7216-4472-4.
- ↑ ŠVÍGLEROVÁ, Jitka. Albumin [online]. Poslední revize 2009-02-18, [cit. 2010-10]. <https://web.archive.org/web/20160416224413/http://wiki.lfp-studium.cz/index.php/Albumin>.
- ↑ RACEK, Jaroslav, et al. Klinická biochemie. 2. vydání. Praha : Galén, 2006. 329 s. s. 71. ISBN 80-7262-324-9.
- ↑ Jump up to: a b ZIMA, Tomáš, et al. Laboratorní diagnostika. 2. vydání. Praha : Galén a Karolinum, 2007. 906 s. ISBN 978-80-246-1423-6.
- ↑ KESSLER, Siegfried. Laboratorní dagnostika. 1. vydání. Praha : Scientia medica, 1993. 252 s. Memorix; s. 52. ISBN 80-85526-12-3.
- ↑ KESSLER, Siegfried. Laboratorní dagnostika. 1. vydání. Praha : Scientia medica, 1993. 252 s. Memorix; s. 52. ISBN 80-85526-12-3.
- ↑ ZIMA, Tomáš, et al. Normální hodnoty [online]. Velký lékařský slovník online, [cit. 2020-02-13]. <http://lekarske.slovniky.cz/normalni-hodnoty>.
- ↑ GREGOR, Pavel a Petr WIDIMSKÝ, et al. Kardiologie. 2. vydání. Praha : Galén, 1999. 595 s. s. 168. ISBN 80-7262-021-5.
- ↑ KLENER, Pavel, et al. Vnitřní lékařství. 3. vydání. Praha : Galén a Karolinum, 2006. 1158 s. ISBN 80-7262-430-X.
- ↑ LIU, H. H., J. B. GUO a Y. GENG. Procalcitonin: present and future. Irish Journal of Medical Science (1971 -). 2015, roč. 3, vol. 184, s. 597-605, ISSN 0021-1265. DOI: 10.1007/s11845-015-1327-0
- ↑ Jump up to: a b c ÚKBLD 1. LF a VFN Praha. Prokalcitonin : vývoj názorů na interpretaci [online]. ©2009. [cit. 2011-06-30]. <http://www.cskb.cz/res/file/akce/sjezdy/2009-Pha/ppt/B1/Kazda.pdf>.
- ↑ STRICKLAND, RD, ML FREEMAN a FT GURULE. Copper binding by proteins in alkaline solution. Analytical chemistry [online]. 1961, vol. 33, no. 4, s. 545-552, dostupné také z <https://pubs.acs.org/action/cookieAbsent>. ISSN 0003-2700. DOI: 10.1021/ac60172a019.



