Burn Injury
Burn injury is damage to the skin caused by heat (scalding, contact with a heat source or fire), chemicals, electric current or radiation. In children, it is most often skin damage caused by heat. In more severe cases, burn disease develops, which threatens the patient's life initially with burn shock' and later with sepsis.
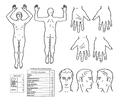
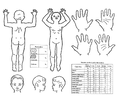
Severity depends on the extent, location, degree of damage and cause of the burn injury. Other important factors are the age and comorbidities of the affected person. In children under 2 years, a severe area affecting > 5% of the body surface area (TB), in children aged 2–10 years > 10% of TP and in children over 10 years > 20% of TP. burns on the face, hands, feet, perineum, buttocks and genitals are serious. Electric shock is serious regardless of the extent of the burned area and location. The degree of skin damage depends on the temperature to which it is exposed and the duration of exposure. The extent of the affected body surface can be estimated according to the so-called rule of nine.[1]
Degrees of burns[edit | edit source]
First degree burns (redness – erythema)[edit | edit source]
Only the epidermis is affected. It is manifested by redness and swelling, blisters do not form. Furthermore, there is increased skin sensitivity and pain, as there is a reaction of macrophages at the burn site, which produce cytokines, which stimulate nociceptors after penetrating the dermis. Changes are reversible, healing occurs spontaneously after a few days. The mechanism of spontaneous treatment is represented by the stem cells of the basal layer of the epidermis, which divide mitotically multiple times. No scar is formed. The epidermal barrier remains intact, therefore the metabolic response and the risk of infection are minimal.
Second degree burns - superficial (IIa; blister - bula)[edit | edit source]
The damage involves the epidermis and the surface part of the dermis (papillary layer). The deep part of the dermis remains vital and allows the regeneration of the epithelium in the wound area. Thin-walled blisters filled with fluid (leaking plasma from the capillaries at the interface of the dermis and epidermis) are formed, which are very sensitive to touch. The wound will heal spontaneously within 2-3 weeks. Re-epithelialization usually occurs within 7–10 days after the injury, by the growth of "buds" of epithelium from vital pilosebaceous units and sweat glands from the papillary and reticular part of the dermis. It usually heals without scarring.
Second degree burns - deep (IIb)[edit | edit source]
The damage extends to the deep (reticular) layer of the dermis. The skin is red and whitish, capillary filling is slowed down. The blisters are thick-walled. 2-point discrimination may be impaired, but sensitivity to pressure and pain is preserved. Spontaneous epithelialization may occur from vital parts in the deepest layers of the skin. Healing usually occurs within 3-6 weeks. These wounds have a higher risk of hypertrophic scarring. Antimicrobial dressings are suitable for treatment to prevent infection. Contractures may occur in the joint area, causing limited mobility.
Third degree burns (necrosis)[edit | edit source]
This is irreversible damage to the epidermis and dermis in its entire width, destruction of the capillary network of the dermis. The wound has a white or gray-white color. The wounds are painless. Very small areas can heal by contraction of the surrounding tissue, small and larger areas require a skin graft.
Fourth degree burns (chars)[edit | edit source]
Charring is damage to the skin and subcutaneous tissue, including damage to muscle fascia, muscles, bones and other structures. Wounds require extensive debridement and complete reconstruction of damaged structures.[2][3][4]
Estimation of extent of disability[edit | edit source]
The so-called rule of nine or tables according to Lund-Browder are used to estimate the percentage of body surface involvement.
Rule of Nine[edit | edit source]
- Head: 9%;
- torso
(front + back): 18 + 18%;
- upper limbs: 2 × 9%;
- lower limbs: 2 × 18%;
- genitalia: 1%.
The younger the child, the larger the proportion of the body surface is the head and the smaller the lower limb. Estimate of the percentage of body surface involvement in children from 1 to 4 years:
- head: 19%;
- torso (front + back): 18 + 18%;
- upper limbs: 2 × 9.5%;
- lower limbs: 2 × 15%;
- genital: 1%[4].
Tables according to Lund-Browder[edit | edit source]
Definition of severe burn in children[edit | edit source]
We determine the degree of severity according to the extent of the burned surface, the location, the character of the burn and the age of the child (the younger the age, the more serious the condition). All the groups listed below belong to the care of the burn center:
| Criteria for a burn center | ||||
|---|---|---|---|---|
| GROUP | 1. | 2. | 3. | 4. |
| AGE (years) | 0–3 | 3–10 | 10–15 | >15 |
| DEGREE (max.) | IIa | IIa | IIa | IIa |
| RANGE (%) | >5 | >10 | >15 | >20 |
Furthermore, there are IIb'' and III burns at any age and extent, and burns with chemicals. Transportation to a burn center is also indicated in case of impact to the face, neck (risk of airway obstruction due to collateral swelling), respiratory tract, genitals and buttocks (risk of infection), hand and foot (risk of permanent deformities) or conditions with suspected or confirmed CO inhalation.
Burn Disease and Burn Shock[edit | edit source]
Burn disease is a generalized reaction to a burn injury that usually develops when more than 10-15% of the TP is affected (5-8% in infants). There is increased capillary permeability, loss of fluids, salts and proteins into the interstitium and subsequently hypovolemic shock. It is accompanied by a high risk of infectious complications.[4]
Pathophysiology[edit | edit source]
Mediators are massively released from burned skin and subcutaneous tissue affected by impaired circulation - leukotrienes, prostaglandins, oxygen radicals, kallikrein, bradykinin, histamine, etc. The permeability of blood vessels increases first at the site of the injury and its surroundings, then in the whole organism as a result of systemic inflammatory response (SIRS). plasma proteins leak out of the vessels, reducing the intravascular oncotic pressure. Fluids leak through the damaged skin to the surface and simultaneously accumulate in the burn swelling. Effective circulating blood volume rapidly decreases and burn shock develops.[5]
During the first day after a more severe burn injury, three concentric zones of tissue damage can be distinguished[6]:
- central coagulation zone
- Occurs at the point of most intense contact with the heat source. It is formed by dead and dying cells due to coagulation necrosis and lack of blood circulation. It is usually white or tan;
- middle zone of stasis
- The zone is usually red and may blanch when pressed, making its circulation appear undamaged, however, within 24 hours there is usually a stoppage of circulation through the superficial vessels. Petechial bleeding is also common. Whitening occurs by the third day after the injury due to the death of the avascular surface layer of the dermis. Experimental studies show that gradual vascular occlusion is caused by flushed prostaglandins, histamine, and bradykinin, which increase the permeability of endothelial cells and the basement membrane, thereby causing edema. The presence of free oxygen radicals (such as xanthine oxidase) is probably involved in the development of edema.
- external zone of hyperemia
- It is red and white when pressed and has an intact blood supply.[7]
In airway burn, the airways swell and ARDS develops. Breathing is affected even with a burn affecting the chest wall like a corset in the entire thickness of the skin.[1]
Systemic inflammatory response[edit | edit source]
In extensive burn injuries (usually involving more than 30% of TP), systemic inflammatory response (SIRS) develops due to the release of cytokines and other mediators into the systemic bloodof circulation. Fluid extravasation occurs as a result of increased vascular permeability in the burned tissue. Fluid losses lead to hypovolemia, which further impairs blood flow and tissue oxygenation. In burned skin, fluid is lost through evaporation, which also causes heat loss. In third-degree burns, hemolysis (due to direct heat damage and reduced erythrocyte half-life due to microangiopathic hemolytic anemia) may occur, requiring transfusions to replace blood loss.
Consequences of SIRS:
- vasoconstriction of the splanchnic and impaired blood flow in internal organs due to released catecholamines, vasopressin and angiotensin;
- deterioration of myocardial contractility due to inflammatory cytokines and tumor necrosis factor alpha;
- impaired lung function due to bronchoconstriction caused by humoral factors such as histamine, serotonin and thromboxane A2.
As a result of reduced circulating volume, cardiac output decreases and peripheral vascular resistance increases. After replacing the lost fluids, the cardiac output increases above the norm. This hyperdynamic state is a reflection of the hypermetabolic phase.
In response to impaired lung function, there is an immediate increased minute volume (increase in respiratory rate and tidal volume). Pulmonary vascular resistance rises, probably due to the release of vasoactive amines and other mediators. Increased pulmonary vascular resistance partially prevents the development of pulmonary edema during volume therapy (when compensating for lost fluids). In the absence of an inhalation injury, there are no changes in the permeability of the pulmonary capillaries.
After a burn injury, glomerular filtration is reduced and renal flow is reduced due to hypovolemia.
After severe burns, there is a hypermetabolic response that can last even a year after the injury and is associated with impaired wound healing, increased risk of infection, loss of body mass, impaired rehabilitation and delayed integration of the affected person into society.[7]
Burn Shock[edit | edit source]
Burn shock accompanies severe burn injuries and is a combination of distributive and hypovolemic shock.[8][9][10] There are losses of circulating plasma volume, hemoconcentration, development of edema, decreased urine production and deterioration of cardiac function.[8][11] Decreased cardiac output is caused by decreased plasma volume, increased afterload and decreased myocardial contractility.[8] The development of hypovolemia is caused by extravasation and loss of electrolytes and proteins into the interstitium as a result of increased vascular permeability at the site of the burn with subsequent reduction of oncotic pressure and evaporation of fluids at the site of damaged skin.[7]
The extent of edema in the burn area peaks 24 hours after the injury. Edema leads to worsening tissue hypoxia and increased tissue pressure. Aggressive volume therapy corrects hypovolemia but worsens the development of edema.[8]
Clinical manifestations of burn shock: tachycardia, hypotension, oliguria.[12] At the beginning of the development of burn shock, blood pressure may be temporarily increased due to hypodynamic shock with high vascular resistance.[5]
Therapy[edit | edit source]
Pre-hospital care[edit | edit source]
Avoiding further exposure to heat is important. We are calling an ambulance. Next, we apply sterile cover to the burned surfaces. We cool the wounds, but the total cooled area should not exceed 10% of TP. We secure intravenous (or bone marrow) access and administer fluids 1/1 Hartmann's solution (or other balanced crystalloid) at a rate of 20-40 mL/kg/h. We monitor blood pressure and urine output.[1][5] We provide analgesia - for children, for example:
- in children > 1 year: tramadol 1–2 mg/kg i.m. (max. 8 mg/kg/24 hours),
- pethidine 0.5–1 mg/kg per IV dose (max. 25 mg per dose),
- ketamine 1 mg/kg i.v./p.r. (max. 50 mg pro dosi) and midazolam 0.1–0.2 mg/kg i.v./p.r. (max. 5 mg for a dose),
ICU therapy[edit | edit source]
We treat burns under general anesthesia, apply a sterile cover and cool. We insert a central venous catheter and continue infusion treatment and analgosedation. It is important to consider the administration of corticosteroids in the first stagesminutes after injury before SIRS is fully developed. We consider tracheal intubation and UPV when airways are affected. We monitor the development of burn swelling on the limbs and neck - as early as 8 hours after the injury, there is a risk that the burn swelling will damage the circulation in the limbs and begin to suffocate the patient by compressing the arteries. We consult with the surgeon about the necessity of releasing incisions. ATB are administered prophylactically or therapeutically [13].. Monitor blood pressure, heart rate, body temperature and diuresis (ECKO indicator - indicator of proper treatment of burn shock). Depending on the situation, we consider transferring the patient to a specialized workplace. We monitor natremia, natriuria and osmolarity of serum and urine (with a good response to treatment, there is a massive return of sodium from the cells and from the fluid sequestered back into the circulation within 18-36 hours). After the end of the burn shock, we start the energy supply - preferably enterally. Surgical treatment – early necroctomy and hetero- or autotransplantation of skin covers.[1]
Volumotherapy[edit | edit source]
The goal of intravenous fluid replacement is to restore and maintain tissue perfusion, thereby preventing organ ischemia, and, conversely, minimizing the development of generalized edema accompanied by pulmonary edema and even more impaired blood supply to the affected areas. Target diuresis is 1 ml/kg body weight/1 hour.
Absolute indications for intravenous fluid replacement are:
- in adults, total involvement of 15% of the body surface,
- in children under 2 years of age, 5% of the body surface is affected,
- in older children, 10% of the body surface is affected.
Fluid losses are the fastest in the first hours, so in the first eight hours about 1/2 of the total amount of fluids calculated for 24 hours is administered.
- In adults, Brook's formula is used for calculation: 3 ml × kg body weight × % impairment.
- In children, it is necessary to take into account the age-specific physiological need for fluids: 2 ml × kg body weight × % disability + (140 − 10×n) × kg body weight; (n = years of age).
Opinions on the most suitable composition of replacement solutions differ. Most often, Hartmann's solution is used, followed by frozen plasma from colloid solutions, the amount of which is corrected according to the values of hypoproteinemia.
When assessing the adequacy of infusion treatment, the following are assessed:
- general circulation (blood pressure and pulse, skin color, body temperature),
- visceral circulation (diuresis, absorption of liquid from the intestine),
- plasma volume (hematocrit and hemoglobin in peripheral blood).
H2 receptor blockers are given to prevent stress gastrointestinal complications. Continuous heparin. In the case of inhalation, preventive antibiotics; a lower diuresis is enough for the risk of pulmonary complications.[12]
Hartmann's solution – isotonic solution of electrolytes, composition: Na 131, K 5, Ca 2, Cl 111, lactate 26 mmol/l; osmolarity 278 mOsm/l; pH 5.0 - 7.0.
Aftercare and rehabilitation[edit | edit source]
We recommend to patients daily lubrication of the affected skin - lubrication 3 times a day and pressure massages (pressing the area for 10 seconds so hard that the area under the pressing finger turns white). Tailored elastic garments are used - pressure on the scar helps to flatten the scarred area. In the case of affected areas where mobility needs to be maintained (joints, neck), we recommend splinting to prevent restriction of movement, splints are usually worn overnight. At night, silicone sheets are inserted under compression sleeves to soften and smooth the scarred areas. It is possible to treat with biostimulation laser[3].
Complications[edit | edit source]
- Infection, sepsis;
- cardiac insufficiency with pulmonary edema;
- respiratory insufficiency (shock lung, sepsis);
- brain edema (with hypoosmolarity of the internal environment);
- gastrointestinal bleeding (stress), paralytic ileus;
- renal insufficiency (kidney shock with hypovolemia);
- formation of keloid scars, formation of contractures[4].
References[edit | edit source]
Related Articles[edit | edit source]
External links[edit | edit source]
- Initial professional treatment of burn trauma Recommended procedure - pre-hospital care for thermal injury
Update: 2017 (edited 2019)
- Burn — interactive algorithm + test
- Posttanning with heat, cold and electric current - recorded lectures 1.LF
- Solving an emergency with a large number of patients with thermal injury Interdisciplinary recommended procedure 2020
- Presentation of B.Bachelor Ostrava - Burn trauma IPVZ
- Factors of the severity of burn injury in childhood, R.Zajíček, I.Grosová, H.Šuca Department of Burn Medicine FNKV and 3rd Faculty of Medicine UK, Prague
References[edit | edit source]
- ↑ Jump up to: a b c d NOVÁK, Ivan, et al. Intenzivní péče v pediatrii. 1. edition. Praha : Galén, 2008. pp. 537-543. ISBN 978-80-7262-512-3.
- ↑ EDLICH, RF. Thermal Burns : Quantifying Burn Severity [online]. Medscape, ©2013. [cit. 2014-02-09]. <https://emedicine.medscape.com/article/1278244-overview>.
- ↑ Jump up to: a b Sdružení na pomoc popáleným dětem. Popáleniny a hlavní zásady jejich léčby [online]. Sdružení na pomoc popáleným dětem, ©2008. The last revision 2008-01-08, [cit. 2011-01-29]. <http://popaleniny.cz/>.
- ↑ Jump up to: a b c d MUNTAU, Ania Carolina. Pediatrie. 4. edition. Praha : Grada, 2009. pp. 540. ISBN 978-80-247-2525-3.
- ↑ Jump up to: a b c NOVAK, J. Burn injuries in children. Pediatrics for practice [online]. 2006, y. -, vol. 2, p. 89-91, Available from <http://www.solen.sk/index.php?page=pdf_view&pdf_id=1492>.
- ↑ Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953;40:588.
- ↑ Jump up to: a b c EDLICH, RF. Thermal Burns : Pathophysiology [online]. Medscape, ©2013. [cit. 2014-02-09]. <https://emedicine.medscape.com/article/1278244-overview>.
- ↑ Jump up to: a b c d LATENSER, BA. Critical Care of the Burn Patient: The First 48 Hours. Crit Care Med [online]. 2009, y. 37, vol. 10, p. 2819-2826, Available from <https://login.medscape.com/login/sso/getlogin?urlCache=aHR0cHM6Ly93d3cubWVkc2NhcGUuY29tL3ZpZXdhcnRpY2xlLzcxMTQzOA==&ac=401>.
- ↑ Ahrns KS: Trends in burn resuscitation: Shifting the focus from fluids to adequate endpoint monitoring, edema control, and adjuvant therapies. Crit Care Nurs Clin N Am 2004; 16:75–98
- ↑ Ipaktchi K, Arbabi S: Advances in burn critical care. Crit Care Med 2006; 34:S239–S244
- ↑ Barton RG, Saffle JR, Morris SE, et al: Resuscitation of thermally injured patients with oxygen transport criteria as goals of therapy. J Burn Care Rehabil 1997; 18:1–9
- ↑ Jump up to: a b KAPOUNKOVÁ, Z. Burn Shock. ZDN [online]. 2001, y. -, vol. 24, p. -, Available from <https://web.archive.org/web/20160331222721/http://zdravi.e15.cz/clanek/priloha-lekarske-listy/popaleninovy-sok-136676>.
- ↑ HANUŠ, Rozsypal,. Infekční nemoci ve standardní a intenzivní péči. - edition. Charles University in Prague, Karolinum Press, 2013. 396 pp. ISBN 9788024621975.