Congenital thrombophilia
Thrombophilia (hypercoagulable state) is a condition characterized primarily by a susceptibility to increased formation of thrombus. Then can occur thrombosis, which is the intravital formation of a blood clot (thrombus) in the heart or in the blood vessels, or "thromboembolism", when a thrombus is carried by the bloodstream to a distant location. This is where the clot becomes trapped and has functional consequences for proper blood flow. Another symptom of thrombophilia is an increased number of platelets.
The severity of thrombophilia is determined based on the specific mutation (some are more aggressive) and whether the patient is diseased in a homozygous or heterozygous constitution of alleles.
The clinical symptom of "congenital thrombophilia" is repeated venous thrombosis, most often in the lower limbs (mainly in the calves) and thromboembolism to the lungs. These symptoms are increasingly occurring in young people, due to the use of hormonal contraception in women, smoking and a sedentary lifestyle. In recent times is also common the penetration of a thrombus into the brain leading to a cerebral stroke, with all its consequences.
Etiology[edit | edit source]
Congenital thrombophilia has several causes:
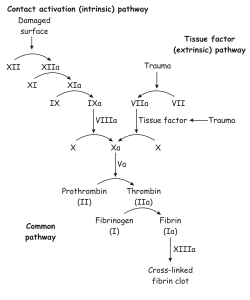
- congenital deficiency or reduced function of antithrombin III,
- deficiency of proteins C and S,
- deficiency of fibrinogen,
- resistance factor V (so-called Leiden mutation),
- disorder of fibrinolysis (deficiency of plasminogen, tPA, excess PAI-1),
- deficiency of faktoru VIII (so-called Von Willebrand disease).
Factor V resistance[edit | edit source]
It is the most common cause of hypercoagulability (prothrombic state). During the mutation of "factor V", there is resistance to proteolytic cleavage by activated protein C. Usually, there is an exchange of the amino acid arginine for glutamine in the molecule. This mutation is called Leiden mutation. Heterozygotes comprise about 3% of the population and the risk of thromboembolic disease is about 7× higher than in the healthy population. If it is a population of homozygotes, the risk of thromboembolic disease increases up to 20x. The risk increases many times (even 100 times) while using hormonal contraception, smoking and having a sedentary lifestyle.
Congenital deficiency or reduced function of antithrombin III[edit | edit source]
A risk factor is considered to be a 'reduction in the level of antithrombin III below 50%, which also applies to heterozygotes who have one defective allele. Some mutations are characterized by reducing the effectiveness of this factor in inhibiting factor Xa and IIa. These factors reduce the binding between antithrombin III and heparin, antithrombin's activation by heparin, or its serine protease activity. Homozygotes have it more difficult in that heparin is thought to be ineffective in reducing blood clotting. Antithrombin III is necessary for a well heparin's reaction.
Lack of proteins C and S[edit | edit source]
A lack of those proteins causes autosomal dominant (AD) character of the disease, and that's why the disease manifetst itself already when only one allele is disabled. Phenotypically, this deficiency almost always manifests as recurrent venous thrombosis or pulmonary thromboembolism. Medicines based on heparin' are used for treatment rather than medicines based on warfarin because warfarin further reduces the effect of [[Vitamin K] ], which could lead to a further reduction of these proteins and support the development of thromboembolic disease.
Lack of fibrinogen (dysfibrinogenemia)[edit | edit source]
If there is a mutation in some of the chains that make up the fibrinogen molecule, it is likely that its conversion to fibrin by factor IIa (thrombin) will be reduced and a 'total clotting disorder will occur..
Disorder of the fibrinolysis[edit | edit source]
Fibrinolysis is a process during which a solid thrombus is transformed into a liquid state, i.e. it dissolves. Fibrinolysis is the result of the cleavage of fibrin by the proteolytic enzyme plasmin. Plasmin is formed in a clot from plasminogen. For plasminogen to be converted to effective plasmin, there must be present tissue plasminogen activator (tPA) and urokinase-type activator (uPA). In the case of thrombophilia, it is therefore an insufficient formation or release of tPA and resistance of plasminogen to its activation via tPA and uPA.
Dispensary[edit | edit source]
In general, a sedentary lifestyle promotes cardiovascular disease and thus the development of thromboembolic disease. Patients who are suspected of having congenital thrombophilia will most often go to a hematology clinic for sampling, where they will have genetic tests paid for by the insurance company. These tests detect the most common mutations, which include 'factor V Leiden mutation, MTHFR mutation and factor VIII mutation. When one of these mutations is detected, it is paid attention whether the patient is homozygous or heterozygous for the given mutation.
If, for example, there is heterozygous for the MTHFR (methyltetrahydrofolate) mutation, we can say with quite a lot of certainty that the patient has a low probability of developing thromboembolic disease. If the patient has a mutant factor V Leiden, and even in a homozygous constitution, the probability of thromboembolic disease is high.
When congenital thrombophilia is detected, the patient in the Czech Republic receives a card with the inscription CAUTION! RISK OF THROMBOSIS (czech: POZOR! RIZIKO TROMBÓZY!). This card also has information about the place where the card was issued, basic information about the patient and the name of the mutation. Furthermore, contraindications are listed there. The patient always carries this card with him in his wallet.
In the case of immobilization (extremity fixation, laiding up in bed , surgery, long journeys by means of transport, transoceanic flights, etc.), the patient applies preventive anticoagulant treatment with low molecular weight heparin. Clexane or zibor are most often prescribed. This anticoagulant treatment is also recommended in case of dehydration, in the 3rd trimester of pregnancy, six months of pregnancy or during hormonal treatment. These anticoagulants are in the form of injections, which are most often injected into the abdomen (into the fat layer). Restrictions also apply to certain foods, patients should not eat spinach, cabbage, brussel sprouts and other foods rich in vitamin K.
Source[edit | edit source]
Related articles[edit | edit source]
- Pulmonary embolism
- Heparin
- Leiden mutation
- Thromboembolic disease in gynecology
- Thromboembolic disease (pediatrics)
- Platelet plug
Links[edit | edit source]
Source[edit | edit source]
- POVÝŠIL, Ctibor – ŠTEINER, Ivo, et al. Obecná patologie. 1. edition. Praha : Galén, 2011. pp. 290. ISBN 978-80-7262-773-8.
- NEČAS, Emanuel. Patologická fyziologie orgánových systémů : Část I. 2. edition. V Praze : Karolinum, 2009. pp. 379. ISBN 978-80-246-1711-4.