Hemoglobin and its derivatives (LF MU)
Hemoglobin is a red blood pigment that ensures the transport of oxygen from the lungs to the tissues and the transport of CO 2 and protons from the peripheral tissues to the respiratory organs.The concentration of hemoglobin in a healthy adult man is approximately 150 g/l, in an adult woman about 140 g/l. One gram of hemoglobin can bind up to 1.34 ml of oxygen. [1]
The structure of hemoglobin[edit | edit source]
It is a tetrameric protein made up of four subunits. Always two and two subunits are identical. In physiologically occurring hemoglobins, there are four types of polypeptide chains α, β, γ, and δ, which differ in the number and sequence of amino acids . The tetramer is made up of two α chains and two other types of chains that give the character of the entire hemoglobin molecule. In adults, hemoglobin A predominates, the structure of which includes, in addition to two α chains (141 amino acids), two β chains (146 amino acids).
Each subunit includes a polypeptide chain to which one heme is covalently bound . The basis of the heme molecule is protoporphyrin, formed by 4 pyrrole nuclei connected by methenyl bridges with centrally bound iron. Heme iron is six-bonded overall – it is connected to the nitrogen atoms of the pyrrole nuclei by four coordination bonds. Another coordination valency binds iron to the imidazole group of the amino acid histidine in the globin chain. The sixth valency of Fe is assigned to the oxygen molecule (O 2 ).
Hemoglobin in the blood[edit | edit source]
Determination of hemoglobin in the blood is one of the most basic laboratory tests. The concentration of hemoglobin in the blood is the main criterion for assessing whether it is anemia . The term anemia is used when hemoglobin or erythrocytes fall below the lower limit of physiological values. Anemia is one of the most common clinical findings. It is a condition that leads to a decrease in the binding capacity for oxygen and a subsequent disorder of tissue respiration.
Causes of anemia[edit | edit source]
Anemia occurs when erythropoiesis is unable to meet the requirements for the production of new red blood cells. It develops as a result of blood loss or increased destruction of red blood cells or insufficient production of red blood cells. The following overview lists some specific causes of anemia:
- Anemia from increased blood loss :
- Acute blood loss.
- Chronic blood loss.
- Anemia from increased breakdown of erythrocytes (hemolytic states).
- Autoimmune hemolytic anemia (presence of antibodies against one's own erythrocytes).
- Erythrocyte membrane disorder (deviation in the composition of the erythrocyte membrane).
- Hereditary enzyme defects of erythrocytes (pyruvate kinase, glucose-6-phosphate dehydrogenase).
- Unstable hemoglobins – hemoglobinopathy (e.g. hemoglobin S in sickle cell anemia).
- Anemia from reduced erythrocyte production .
- Lack of substances needed for erythropoiesis (lack of iron, lack of vitamin B12, lack of folic acid, lack of erythropoietin - chronic renal diseases, lack of other substances, e.g. vitamins B1, B6).
- Anemia due to chemical, physical and radiation damage.
- Anemia in chronic inflammatory, infectious and tumor diseases.
An increase in hemoglobin values can be a manifestation of dehydration of the organism or chronically reduced pulmonary ventilation. Rarely, it can be caused by some myeloproliferative conditions, eg polycythemia vera .
Principle of determination of hemoglobin in blood[edit | edit source]
Oxidation of hemoglobin to methemoglobin:
| HbFe II | + | [Fe III (CN) 6 ] 3− | → | HbFe III | + | [Fe II (CN) 6 ] 4− |
| Hemoglobin | Methemoglobin |
Conversion of methemoglobin to cyanomethemoglobin:
| HbFe III | + | CN − | → | HbFe III CN | |
| Methemoglobin | Cyanmethemoglobin |
The photometric determination is based on the oxidation of divalent iron in hemoglobin by potassium hexacyanoferrate to trivalent iron. In further reaction with potassium cyanide, the resulting methemoglobin is converted into very stable cyanomethemoglobin with a single broad absorption maximum in the visible region at 540 nm.
Evaluation: The reference range of hemoglobin concentration in the blood (hemoglobin B) for an adult man is 130-180 g/l and for a woman 120-160 g/l.
Task: Determination of hemoglobin in blood (pdf)
Hemoglobin in urine[edit | edit source]
Up to a million erythrocytes per day are excreted in the urine of completely healthy people. This very small quantity cannot be demonstrated by ordinary chemical tests. The occurrence of a large number of erythrocytes ( hematuria , erythrocyturia) or penetration of free hemoglobin, or of muscle myoglobin into definitive urine ( hemoglobinuria , or myoglobinuria) is almost always a pathological finding. Macroscopic hematuria is observed with the naked eye; urine has a pinkish color (comparable to water from washed meat) and hemoglobin can be detected in it spectroscopically. Urine contains at least 1 g of hemoglobin per liter. In massive hemoglobinuria, the urine can even have a dark beer color (degradation of hemoglobin to hematin ). Microscopic hematuriait is demonstrable only biochemically.
Determination of hemoglobin in urine[edit | edit source]
Hemoglobin catalyzes, similarly to peroxidase , the oxidation (dehydrogenation) of some substrates (e.g. benzidine derivatives ) by hydrogen peroxide:
However, it is not an enzyme activity (catalysis is conditioned by heme iron), and therefore it is not lost even after thermal denaturation. We are talking about pseudoperoxidase activity , which is used for sensitive but non-specific evidence of hemoglobin or trace amounts of blood. It is advantageous to use a chromogenic substrate to monitor the reaction, i.e. a substance that gives a distinctly colored product by dehydrogenation (often benzidine or its non-carcinogenic derivatives, aminophenazone, etc.).
The reagent zone of diagnostic strips contains a chromogen (e.g. tetramethylbenzidine ) with stabilized hydrogen peroxide (e.g. cumene hydroperoxide ). In the presence of free hemoglobin ( hemoglobinuria ), the indicator zone becomes uniformly blue. If erythrocytes are present in the urine ( erythrocyturia ), intensely greenish-blue colored dots or spots are formed.
Hemoglobinuria can be seen in intravascular hemolysis . Damage to the glomerular membrane ( glomerular hematuria ) and bleeding from any part of the urinary tract lead to more frequent erythrocyturia. It is often found in urinary tract infections , urolithiasis and tumors of the urogenital tract .
In addition to hemoglobin, myoglobin also provides a pseudoperoxidase reaction , which can be excreted in the urine during the breakdown of skeletal muscle ( rhabdomyolysis , crush syndrome ). The positivity of the test can also be caused by peroxidases of leukocytes or some bacteria, yeasts or fungi, which can occur in urine, especially during urinary tract infections . If we want to exclude the possibility of a false positive reaction due to the effect of cellular peroxidases, the reaction must be performed with boiled urine.
Contamination of the collection vessel with strong oxidizing agents also causes a false positive reaction. On the other hand, the presence of strongly reducing substances (e.g. ascorbic acid ) can slow down or stop the pseudoperoxidase reaction and thus be the cause of false negative results.
Assignment: Detection of blood and blood dye in urine (pdf)
Hemoglobin in the stool - occult bleeding[edit | edit source]
Evidence of occult (hidden) bleeding is used to detect the early stages of colorectal cancer , when radical and effective treatment is possible. The examination consists of capturing traces of blood in the stool , various methodological procedures are used:
- The methods use the pseudoperoxidase activities of hemoglobin. The patient must follow a diet for 3 days before the examination, exclude uncooked meat, salami, bananas, tomatoes from the diet, must not take medicines containing ascorbic acid or acetylsalicylic acid. After that, the patient himself takes samples from three consecutive stools and applies them to the test cards. The evaluation is carried out in the laboratory, the principle is similar to that of hemoPHAN diagnostic strips
- Other methods are based on the immunochemical detection of hemoglobin using an antibody against human hemoglobin. Immunochemical methods are more sensitive and specific, there is no need to keep a diet before the examination. Positive results must be verified by other diagnostic methods.
Task: Test for occult bleeding in the digestive tract (pdf)
Hemoglobin derivatives[edit | edit source]
Hemoglobin derivatives include the following types:
Oxyhemoglobin and deoxyhemoglobin[edit | edit source]
Oxyhemoglobin and deoxyhemoglobin
Hemoglobin carrying oxygen is referred to as oxyhemoglobin (oxyHb) . Each Hb molecule can bind 4 oxygen molecules. After the release of oxygen, we speak of deoxyhemoglobin (deoxyHb) . In both forms, iron is divalent, as only hemoglobin containing Fe II+ can reversibly bind and transport an oxygen molecule. Oxygenation of the hemoglobin molecule changes the electron state of the Fe II+ -heme complex, which is manifested by a change in the color of the dark red shade typical of venous blood to the bright red color of arterial blood. In the human body, about 98.5%[2] of oxygen is bound to hemoglobin.
Carbaminohemoglobin[edit | edit source]
Carbaminohemoglobin is hemoglobin to which CO 2 is bound . Carbon dioxide binds to the globin chain of hemoglobin . The binding of CO 2 to hemoglobin reduces the affinity of hemoglobin for oxygen.
Methemoglobin[edit | edit source]
Methemoglobin (metHb; also hemiglobin or ferrihemoglobin ) is characterized by the presence of trivalent iron , which is formed by the oxidation of divalent iron in hemoglobin[3]. Methemoglobin loses the ability to reversibly bind oxygen . In its place, Fe III+ binds a water molecule with a sixth coordination bond. The color of methemoglobin is chocolate brown.
Methemoglobin is present in erythrocytes in small amounts even physiologically (about 1–3% of the total hemoglobin concentration[4]). This is mainly due to the effect of nitrites, which arise from nitrates contained in food. The reverse reduction of methemoglobin to hemoglobin is primarily ensured by the NADH-dependent cytochrome-b 5 reductase (also methemoglobin reductase). A minor role is played by NADPH-dependent methemoglobin reductase, which is dependent on the supply of NADPH from the pentose cycle and on the presence of another electron carrier (e.g. flavin)[5]. Non-enzymatic mechanisms include the action of glutathione and ascorbic acid .
An increased concentration of methemoglobin in the blood is referred to as methemoglobinemia . The causes are various:
- Hereditary methemoglobinemia is usually caused by a congenital defect in NADH-dependent methemoglobin reductase or the presence of abnormal hemoglobin M.
- Acquired methemoglobinemia is the most common form of methemoglobinemia. It can be caused by the action of oxidizing substances:[6]
- poisoning by certain substances ( nitrobenzene , aniline and its derivatives - e.g. some dyes),
- under the influence of certain drugs (local anesthetics – benzocaine , phenacetin , sulfonamides ),
- increased content of nitrates and nitrites in water and food.
Newborns are especially sensitive to the increased content of these substances due to the immaturity of the reduction systems and the increased proportion of fetal hemoglobin, which is more easily oxidized. Methemoglobinemia is manifested by cyanosis with a characteristic gray-brown shade and hypoxia.
| Methemoglobin values | Symptoms |
|---|---|
| 0-2% | normal value |
| < 10% | cyanosis |
| < 35% | cyanosis and other symptoms (headache, shortness of breath) |
| 70% | lethal concentration |
Part of the therapy of acquired methemoglobinemia is the administration of some reducing agents - methylene blue or ascorbic acid.
Carbonylhemoglobin[edit | edit source]
Carbonylhemoglobin (COHb, carboxyhemoglobin ) is formed by the binding of carbon monoxide to hemoglobin . The bond formed is 250–300 times stronger than that of oxygen . Carbonylhemoglobin cannot transport oxygen, and cellular hypoxia develops as a result of the blood's reduced ability to carry oxygen . In an excess of oxygen, the binding of carbon monoxide to hemoglobin is reversible. That is why inhalation of O 2 is most important in carbon monoxide poisoning .
Small amounts of COHb can also occur in healthy individuals. Values of around 2% are found in urban dwellers, and COHb can rise up to 10% of total hemoglobin in heavy smokers . A few minutes' stay in an environment containing 0.1% CO can increase the concentration of carbonyl hemoglobin to 50%.
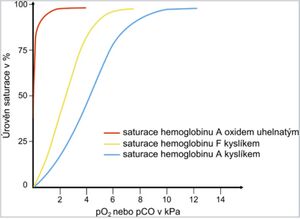
Carbon monoxide is produced during incomplete combustion of fuels, it is also contained in exhaust gases and in smoke during fires in closed rooms. Hemoglobin saturation curve
| COHb values in % | Symptoms |
|---|---|
| 10 | shortness of breath with greater exertion |
| 20-40 | headaches , shortness of breath, fatigue, vomiting |
| 40–60 | hyperventilation, tachycardia, syncope, convulsions |
| 60–80 | coma, death |
Carbonylhemoglobin is characterized by a crimson red coloration; also people with severe carbon monoxide poisoning have a "healthy" pink skin color. Compared to hemoglobin, carbonylhemoglobin is more resistant to chemical influences, it changes more slowly under the influence of various agents.
Spectrophotometry of hemoglobin derivatives[edit | edit source]
Absorption spectra of hemoglobin and its derivatives Hemoglobin and its derivatives have characteristic absorption spectra in the visible light region, which are used for their analysis and rapid identification. Significant absorption maxima in the 400–430 nm region, the so-called Soret band, are typical for all hemoproteins. Other absorption peaks are considerably lower. Oxyhemoglobinis characterized by two incompletely separated maxima in the region of 540 and 578 nm. Deoxyhemoglobin has a single absorption maximum at 555 nm. The main absorption maximum of methemoglobin is at 630 nm and a second faint peak at 500 nm is pH dependent. When methemoglobin reacts with potassium cyanide, the maximum at 630 nm disappears, as cyanmethemoglobin is formed. The decrease in absorbance at 630 nm is proportional to the methemoglobin concentration. Cyanmethemoglobin shows a broad absorption maximum at 540 nm, which is used in determining the concentration of hemoglobin in the blood. The spectrum of carbonylhemoglobin resembles that of oxyhemoglobin, but the position of the peaks is shifted towards lower wavelengths.
| Hemoglobin derivative | Absorption maxima [nm] |
|---|---|
| Hemoglobin reduced | 431, 555 |
| Oxyhemoglobin | 414, 540, 578 |
| Methemoglobin | 404, 500, 630 |
| Carbonylhemoglobin | 420, 538–540, 568–569 |
| Cyanmethemoglobin | 421, 540 |
Determination of carbonyl hemoglobin:
The determination of carbonylhemoglobin in the blood is one of the basic toxicological examinations. It is an objective criterion in the assessment of acute and chronic carbon monoxide poisoning.
- Spectrophotometric evaluation . Carbonylhemoglobin can be determined quickly spectrophotometrically based on reading the shift of the absorption maximum of diluted blood from 586 nm[7]. The shift of the maximum in the spectrum is dependent on the ratio of COHb and O 2 Hb in the sample.
- Reaction with tannin . As a guide, carbonylhemoglobin can be determined by reaction with tannin or Ajatin (from about 10% COHb). Tannin forms a strawberry-red precipitate in the presence of carbonylhemoglobin. In the absence of carbonyl hemoglobin, the color of the precipitate is brownish gray.
- Acid-base balance analyzers . The analysis of the toxicologically most important derivatives of hemoglobin COHb and metHb is also made possible by modern acid-base balance analyzers that have a built-in photometric system for their measurement.
Task: Spectrophotometric examination of hemoglobin and its derivatives (pdf)
Task: Indicative determination of carbonylhemoglobin (pdf)
Glycated hemoglobin HBA1[edit | edit source]
Glycated hemoglobin is produced by a non-enzymatic reaction between hemoglobin and blood glucose . His work is irreversible.
The level of glycated hemoglobin therefore reflects the concentration of glucose in the blood throughout the life of the erythrocyte , i.e. about 120 days , and is used to assess the success of the treatment/compensation of diabetes in the period 4-8 weeks before the examination. The form of the stable fraction HBA 1c is most often determined .
- Terminology
- Glycated hemoglobin – the sum of carbohydrate adducts at the N-terminal end or ε amino groups of lysine in hemoglobin.
- HbA 1 - the sum of various minor fractions of hemoglobin (glycated), including HbA 1c , HbA 1a1/a2 , HbA 1b1/b2/b3 , HbA 1d1/d2/d3 and HbA 1e .
- HbA 1c – valine glucose adduct at the N-terminal end of β-globin; corresponds to the so-called stable ketoamine (N-[1-deoxyfructosyl]hemoglobin).
Glycated hemoglobin can be determined using ion exchange chromatographyfollowed by spectrophotometry .
- Evaluation
- The amount of glycated hemoglobin is expressed in % of total hemoglobin or now in mmol/mol according to the IFCC (International Federation of Clinical Chemistry).
- Reference limits
- in healthy adults up to 39 mmol/mol[8], (2.8–4.0%)
- in diabetics, HbA 1c concentrations up to 45 mmol/mol (4.5%) indicate excellent diabetes compensation, up to 60 mmol/mol (6.0%) acceptable, and higher values indicate unsatisfactory diabetes compensation[9]
Task: Determination of glycated hemoglobin (pdf)
Iron[edit | edit source]
Iron is one of the most important elements in the human body. The body of an adult contains more than 70 mmol (4.0-4.5 g) of iron. In women, this amount is lower than in men, which is attributed to blood loss during menses .
| Form | Function | Protein | Quantity according to |
|---|---|---|---|
| Active iron | oxygen transport | hemoglobin | 2.5-3.0 |
| myoglobin | 0.3 | ||
| electron transfer | cytochromes , cytochrome oxidase | 0.2 | |
| decomposition of hydrogen peroxide | catalase , peroxidase | ||
| Stock iron | ferritin , hemosiderin | 0.8–1.0 | |
| Transport iron | transferrin | 0.003 | |
Iron metabolism[edit | edit source]
The presence of iron is essential for cell function. As a component of heme, it participates in oxygen transport and as a component of cytochromes, it conditions the transfer of electrons in the respiratory chain. An undesirable effect of iron as a transient and very reactive element is participation in radical reactions, during which the so-called reactive forms of oxygen are formed . These can damage cell membranes, proteins and DNA.
Iron is absorbed as Fe 2+ by active transport in the duodenum and upper jejunum in two ways:
- porphyrin-bound Fe in the form of a stable lipophilic complex;
- Fe II+ – chelates soluble in water.
Only a small part is absorbed in ionized form.
There is an average of 10-50 mg of iron per day in the diet, but only 10-15% is absorbed. In heme compounds (meat) it is absorbed better, non-heme Fe in plant food much worse. In addition, plants contain oxalates, phytates, tannins and other phenolic compounds that form insoluble or chelate complexes with Fe that are difficult to absorb. Ascorbic acid, on the other hand, improves iron absorption.
After being absorbed by the intestinal mucosa, part of the iron is incorporated into the storage form - ferritin in the intestinal cells. Part of the absorbed iron passes into the plasma, where it is transported bound to transferrin . The protein ferroportin plays an important role in the transfer of iron across the basolateral membrane of enterocytes (it is also found in the membrane of macrophages and hepatocytes). It is the main place of regulation of iron homeostasis in the organism. A key regulatory factor is the protein hepcidin , which is synthesized in the liver. By binding to ferroportin, it inhibits the transport of iron from cells and thus contributes to its sequestration in them. Hepcidin levels increase during inflammation. Hepcidin is also partly responsible for the anemia of chronic diseases. Mutations in the hepcidin gene lead to juvenile hemochromatosis type 2B .
Plasma iron is taken up by cells of target tissues via the transferrin receptor and is either incorporated into heme or stored in the form of ferritin. The use of the specific transport protein transferrin and the storage protein ferritin for iron storage represent protective mechanisms to prevent the toxic effects of redox-active iron.
During desquamation of dead mucosal cells, unused iron leaves the stool together with unabsorbed iron.
Investigation of iron metabolism[edit | edit source]
In practice, we commonly encounter diseases associated with changes in metabolism and utilization of iron. Laboratory examination of iron metabolism includes the following examinations:
- serum iron
- serum transferrin and iron binding capacity
- serum ferritin
- transferrin receptor
The mentioned parameters are important diagnostic indicators for demonstrating a decrease or increase in iron reserves even in stages that are not accompanied by significant clinical manifestations.
Serum iron determination[edit | edit source]
Colorimetric methods, atomic absorption spectrophotometry and other special techniques are used to determine iron in serum . The most widely used are photometric methods, based on the reaction of iron with a complexing substance. All procedures include the following steps:
- Release of Fe 3+ from binding to transferrin using acids or surfactants (e.g. HCl).
- Reduction of Fe 3+ to Fe 2+ , which is necessary for the reaction with the complexing agent. E.g. ascorbic acid is used for reduction .
- Reaction of Fe 2+ with a complexing agent containing reactive groups –N=C–C=N– to form a colored complex. Metal ions form chelates with two nitrogen atoms. Currently, two complexing substances are mainly used - bathofenthroline and ferrozine (3-(2-pyridyl)-5,6-bis(4-sulfophenyl)-1,2,4-triazine - PST, trademarked name FerroZine®), which has higher absorption coefficient and is more soluble in water.
- Evaluation
- Serum iron concentrations are subject to a circadian rhythm and are influenced by other factors as well. This limits the diagnostic value of this parameter. It is a poor indicator of tissue iron stores and must always be assessed in combination with serum transferrin and iron binding capacity. Reduced concentrations accompany iron deficiency, caused, for example, by large or repeated blood losses, insufficient iron intake through food or impaired absorption. The finding is not specific, as reduced levels are also encountered in acute infections or chronic inflammatory diseases (transfer of iron to tissues). High levels of iron occur in hemochromatosis (see below), iron overdose or intoxication, increased breakdown of erythrocytes, and some liver diseases.
- Reference values
- men: 9–29 μmol/l
- women: 7–28 μmol/l
Serum transferrin and iron binding capacity[edit | edit source]
Iron is transported through the blood bound to a specific protein with β 1 -electrophoretic mobility – transferrin , which is synthesized in the liver. The rate of its formation is inversely proportional to the iron reserves in the body; it increases with iron deficiency and decreases with excess. The biological function of transferrin consists in the ability to easily form non-toxic complexes with iron and transfer Fe absorbed by the mucosa of the small intestine to the bone marrow or to storage forms (ferritin or hemosiderin). Each transferrin molecule binds two Fe 3+ atoms (1 g of transferrin binds 25.2 μmol of iron). Transferrin can be determined directly using immunochemical methods or indirectly as the ability of transferrin to bind iron - the so-called iron binding capacity.Total iron binding capacity ( TIBC ) is the amount of iron that transferrin is able to bind if all binding sites are occupied. Usually, only 1/3 of transferrin is saturated with iron - the bound capacity . Free transferrin without bound iron represents the free binding capacity (2/3 of transferrin) available for iron transport under increased demands.
Conversion between transferrin concentration and total binding capacity:
- Total binding capacity [μmol/l] = transferrin [g/l] · 25.2 .
The reference range for serum transferrin concentration (S-transferrin) is 2.0–3.6 g/l for a total binding capacity of 50–70 μmol/l.
Transferrin saturation[edit | edit source]
From the values of iron and transferrin concentration we can calculate transferrin saturation (TfS) , which is defined as the ratio of serum iron concentration to the total binding capacity of transferrin for iron. It is a sensitive parameter for detecting latent iron deficiency.
- Extra open brace or missing close brace
Assessment of transferrin saturation
- physiological values: 25-50%
- reduction of saturation in iron deficiency: < 15%
- increase in saturation with excess iron: > 50%
Ferritin and hemosiderin[edit | edit source]
Ferritin is the most important storage protein for iron. The ferritin molecule is adapted to bind a large amount of Fe 3+ in a soluble and non-toxic form for the organism. Ferritin is formed by an outer protein shell of 24 subunits - apoferritin (Mr 440,000), delimiting a cavity in which up to 4,500 iron atoms can be concentrated in the form of iron oxyhydroxide (FeO·OH) n in microcrystalline form with phosphates (FeO·OPO 3 H 2 ). The entry and exit of iron atoms allow the pores between the individual subunits of the shell of the ferritin molecule. Normally, its capacity is used at about 20%. It is stored in cells in the liver, spleen and intestinal mucosa.
In the blood serum, ferritin is found in a very low concentration. Serum ferritin concentrations are a measure of iron stores in the body. Low concentrations indicate depletion of the body's total iron reserve and serve to detect iron deficiency anemia early in its prelatent phase. Increased ferritin concentrations are a concomitant phenomenon of high tissue iron stores. We also encounter them in many patients with liver disease, some malignant tumors (tumor marker) or inflammatory diseases (positive acute phase reactant).
The reference range for serum ferritin concentration (S-ferritin) is 30–300 μg/l for men and 20–120 μg/l for women.
Hemosiderin is another storage protein for iron. It is formed by the aggregation of denatured ferritin with other components. It produces particles 1 to 2 μm in size that are visible under a light microscope when iron staining is used. Hemosiderin contains more iron than ferritin, but due to its poor water solubility, it is difficult to obtain. It is formed when the amount of iron in the body exceeds the storage capacity of ferritin.
Transferrin receptor[edit | edit source]
Iron transported in the blood by transferrin is taken up by cells through a specific transferrin receptor (TfR). At a certain stage of development, it is found on the surface of all cells, but it is most expressed on the surface of precursor cells of the red line in the bone marrow. TfR is a transmembrane protein that consists of two identical subunits joined by a disulfide bond. By separating the extracellular domains of the receptor, the so-called soluble fraction of the transferrin receptor (sTfR) , which can be in the form of a dimer or a monomer, is released into the circulation . Cells respond to reduced iron stores by synthesizing increased amounts of transferrin receptors.
An increase in sTfR is a reliable indicator of iron deficiency for hematopoiesis . Elevated levels of sTfR are found in iron-deficiency anemias or hemolytic anemias . Determination of sTfR is valuable in anemic patients in whom ferritin is elevated due to the acute phase reaction. Determining the concentration of sTfR can also be used in patients with a bone marrow transplant to monitor the progress of erythropoiesis.
Immunochemical methods are used for determination.
Disorders of iron metabolism[edit | edit source]
Iron deficiency (sideropenia)[edit | edit source]
Lack of iron in the body is usually caused by insufficient absorption from the intestine or chronic blood loss. It can result in sideropenic anemia (hypochromic microcytic anemia) , which is one of the most common hematological diseases . However, anemia is usually a late symptom of gradually developing sideropenia. It will show up in the blood count only after the iron has almost completely disappeared. Therefore, it is necessary to detect iron deficiency at an early stage, which is not yet accompanied by anemia.
Based on the determination of the basic parameters of iron metabolism, we distinguish three degrees of deficiency:
- Prelatent iron deficiency is the name for a condition where there is a gradual decrease in stores, but the supply of iron to the erythroblasts of the bone marrow is not yet affected . About half of patients have serum ferritin levels below 12 μg/l.
- With latent iron deficiency, its reserves are essentially exhausted. Ferritin is reduced below the lower limit of the norm and is already accompanied at this stage by a decrease in the level of iron in the serum and a reduced supply to the erythroblasts of the bone marrow. The binding capacity for iron increases. A sensitive indicator of latent iron deficiency is a drop in transferrin saturation below 15%. However, anemia does not yet develop.
- With a manifest iron deficiency, anemia develops with a drop in hemoglobin values below the lower limit of the norm. Iron deficiency anemia is characterized by low serum iron and ferritin, and an increased concentration of transferrin (binding capacity for iron). In hemolytic anemias or iron overload, on the other hand, serum iron is increased, while the total binding capacity for iron is reduced.
| Prelatent iron deficiency | Latent iron deficiency | Manifest iron deficiency |
|---|---|---|
| reduction of stored iron – decrease of ferritin | lack of stored iron – decrease in ferritin | lack of stored iron – decrease in ferritin |
| decrease in serum iron | decrease in serum iron | |
| drop in transferrin saturation below 15% | transferrin drop below 10% | |
| increasing the total binding capacity for iron | increasing the total binding capacity for iron | |
| increase in sTfR | increase in sTfR | |
| decrease in hemoglobin concentration - anemia |
Excess iron[edit | edit source]
The organism is not equipped with an excretory pathway for iron, and therefore, under certain circumstances, excess iron can accumulate in the tissues. Early diagnosis can prevent tissue damage from excess iron. Iron overload usually develops very slowly. We distinguish 3 stages:
- In the stage of prelatent iron excess, its content in the organs increases, but without exceeding their storage capacity.
- During the latent stage of iron overload , the storage capacity of the cells is exceeded, but the function of the organs is not yet damaged, the level of ferritin and the level of iron in the serum increase, and the saturation of transferrin rises above 55%.
- In the stage of manifest iron excess, some organs are already damaged.
| Prelatent iron overload | Latent iron overload | Manifest iron excess |
|---|---|---|
| increasing iron stores - increasing ferritin | increase in iron reserves – increase in ferritin above 300 μg/l | increase in iron stores - increase in ferritin (with severe impairment above 2000 μg/l) |
| increase in serum iron | significant increase in serum iron | |
| increase in transferrin saturation above 55% | increase in transferrin saturation (can exceed 90% in severe cases) |
Hemochromatosis
Accumulation of iron in tissues is related to a disease we refer to as hemochromatosis .
- Primary hemochromatosis is a hereditary disease caused by increased absorption of iron from the intestine. Excess iron is stored in parenchymatous organs such as the liver, heart, pancreas, and adrenal glands. It has a toxic effect on affected organs and disrupts their function by catalyzing chronic reactions leading to the formation of free radicals. The main clinical manifestations are skin hyperpigmentation, hepatosplenomegaly and diabetes mellitus .
- Secondary hemochromatosis can develop as a result of, for example, repeated transfusions, excessive intake of iron-containing preparations or hemolytic anemia. In the biochemical picture, we find increasing levels of ferritin and iron in the serum, the saturation of transferrin increases while it simultaneously decreases.
Iron poisoning[edit | edit source]
Children are at risk of accidental ingestion of large quantities of preparations (lentil-like tablets). The lethal dose for a child is 600 mg. For an adult, an iron intake of 40 mg/kg is toxicologically serious, an intake of 60 mg/kg is fatal .
Symptoms include nausea, vomiting (even vomiting blood), abdominal pain, diarrhea (sometimes bloody). Large fluid losses cause shock, kidney failure, and death. If the patient survives this stage of poisoning, he may fall into unconsciousness, convulsions and liver failure after 12 hours. If he survives even this second phase, the poisoning can leave permanent consequences (intestinal damage).
- Treatment of acute poisoning
- Gastric lavage.
- Administer the chelating agent deferoxamine (5–10 g in 50–100 ml of water) through a nasogastric tube.
- Consider intravenous desferoxanin to flush out absorbed iron. A pink-red complex of deferoxamine with iron appears in the urine. The treatment should be repeated until the urine color returns to normal[10] .
Task: Determination of Fe in serum by colorimetric method (pdf)
Links[edit | edit source]
[edit | edit source]
- Carbonylhemoglobin
- carbaminohemoglobin
- Methemoglobin
- Examination of hemoglobin and it’s metabolism
- Heme
Reference[edit | edit source]
- ↑ ŠVÍGLEROVÁ, Jitka. Hemoglobin [online]. Poslední revize 2009-02-18, [cit. 2010-11-11].
- ↑ KITTNAR, Otomar a ET AL.. Lékařská fyziologie. 1. vydání. Praha : Grada, 2011. 790 s. s. 131. ISBN 978-80-247-3068-4.
- ↑ ŠVECOVÁ, D a D BÖHMER. Vrozená a získaná methemoglobinémia a ich liečba. Časopis lékařů českých. 1998, vol. 137, s. 168-170, ISSN 1803-6597.
- ↑ RICHARD, Alyce M, James H DIAZ a Alan David KAYE. Reexamining the risks of drinking-water nitrates on public health. Ochsner J [online]. 2014, vol. 14, no. 3, s. 392-8, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171798/?tool=pubmed>. ISSN 1524-5012.
- ↑ XU, F, K S QUANDT a D E HULTQUIST. Characterization of NADPH-dependent methemoglobin reductase as a heme-binding protein present in erythrocytes and liver. Proc Natl Acad Sci U S A [online]. 1992, vol. 89, no. 6, s. 2130-4, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC48610/?tool=pubmed>. ISSN 0027-8424.
- ↑ CORTAZZO, Jessica A a Adam D LICHTMAN. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth [online]. 2014, vol. 28, no. 4, s. 1043-7, dostupné také z <https://www.ncbi.nlm.nih.gov/pubmed/23953868>. ISSN 1053-0770 (print), 1532-8422.
- ↑ LEDVINA, M. Rychlé spektrofotometrické stanovení karbonylhemoglobinu v krvi. Biochem Clin Bohemoslov. 1987, vol. 16, s. 493-495, ISSN 0139-9608.
- ↑ ČEŠKA, Richard a Tomáš ŠTULC, et al. Interna. 2. vydání. TRITON, 2022. 870 s. ISBN 978-80-7387-885-6.
- ↑ Doporučený diagnostický a léčebný postup pro všeobecné praktické lékaře. Diabetes mellitus. 2005. Dostupné také z URL <https://www.svl.cz/files/files/Doporucene-postupy-2003-2007/Diabetes-mellitus.pdf>.
- ↑ ŠEBKOVÁ, Sylva. Otrava železem [online]. ©2003. Poslední revize 2003-10-06, [cit. 2021-08-16]. <http://medicina.cz/clanky/5819/34/Otrava-zelezem/>.